Dextromethorphan/quinidine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 20: | Line 20: | ||
*[[Paroxetine]] |
*[[Paroxetine]] |
||
*[[Memantine]] |
*[[Memantine]] |
||
==Contraindications== |
|||
*atrioventricular (AV) block, complete, without implanted pacemaker or at high risk of complete AV block |
|||
*concomitant use with drugs containing [[quinidine]], [[quinine]], or [[mefloquine]] |
|||
*concomitant use with drugs that both prolong the QT interval and are metabolized by CYP2D6 (eg, thioridazine, pimozide); effects on QT interval may be increased |
|||
*concomitant use with MAOIs or use of MAOIs within 14 days; risk of serious, potentially fatal, drug interactions including serotonin syndrome |
|||
*[[heart failure]] |
|||
*hypersensitivity to dextromethorphan (eg, rash, hives) |
|||
*hypersensitivity to quinine, mefloquine, quinidine, or dextromethorphan/quinidine with a history of thrombocytopenia, hepatitis, bone marrow depression or lupus-like syndrome induced by these drugs |
|||
*QT interval, prolonged or congenital long QT syndrome or a history suggesting [[torsades de pointes]] |
|||
Revision as of 16:49, 18 December 2012
 | |
 | |
| Identifiers | |
|---|---|
|
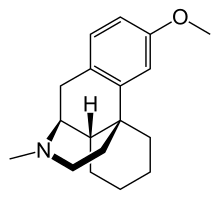
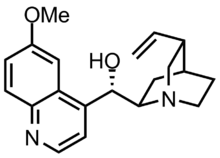
NUEDEXTA is the first FDA-approved drug for the treatment of Pseudobulbar affect. It is a combination product containing dextromethorphan 12 hydrobromide (an uncompetitive NMDA receptor antagonist and sigma-1 13 agonist) and quinidine sulfate (a CYP450 2D6 inhibitor).
Mechanism of Action
Dextromethorphan (DM) is a sigma-1 receptor agonist and an uncompetitive NMDA receptor antagonist. Quinidine increases plasma levels of dextromethorphan by competitively inhibiting cytochrome P450 2D6, which catalyzes a major biotransformation pathway for dextromethorphan. The mechanism by which dextromethorphan exerts therapeutic effects in patients with pseudobulbar affect is unknown.
Pharmacokinetics
NUEDEXTA contains dextromethorphan and quinidine, both of which are metabolized primarily by liver enzymes. Quinidine’s primary pharmacological action in NUEDEXTA is to competitively inhibit the metabolism of dextromethorphan catalyzed by CYP2D6 in order to increase and prolong plasma concentrations of dextromethorphan. Studies were conducted with the individual components of NUEDEXTA in healthy subjects to determine single-dose and multiple-dose kinetics of orally administered dextromethorphan hydrobromide in combination with quinidine sulfate. The increase in dextromethorphan levels appeared approximately dose proportional when the dextromethorphan hydrobromide dose increased from 20 mg to 30 mg in the presence of 10 mg of quinidine sulfate.
Interactions
- Desipramine levels increase 8-fold with co-administration
- Paroxetine
- Memantine
Contraindications
- atrioventricular (AV) block, complete, without implanted pacemaker or at high risk of complete AV block
- concomitant use with drugs containing quinidine, quinine, or mefloquine
- concomitant use with drugs that both prolong the QT interval and are metabolized by CYP2D6 (eg, thioridazine, pimozide); effects on QT interval may be increased
- concomitant use with MAOIs or use of MAOIs within 14 days; risk of serious, potentially fatal, drug interactions including serotonin syndrome
- heart failure
- hypersensitivity to dextromethorphan (eg, rash, hives)
- hypersensitivity to quinine, mefloquine, quinidine, or dextromethorphan/quinidine with a history of thrombocytopenia, hepatitis, bone marrow depression or lupus-like syndrome induced by these drugs
- QT interval, prolonged or congenital long QT syndrome or a history suggesting torsades de pointes
