Uranocene: Difference between revisions
→top: I assume this is what is meant, and not that it's sensitive to green air, or that it is green air and also a sensitive solid |
MagnaGraecia (talk | contribs) correct and improve spectroscopic description |
||
| Line 42: | Line 42: | ||
Uranocene is highly reactive toward oxygen, being [[pyrophoricity|pyrophoric]] in air but stable to [[hydrolysis]]. Considering the molecule to be U<sup>4+</sup>(C<sub>8</sub>H<sub>8</sub><sup>2−</sup>)<sub>2</sub>, the [[hapticity|η<sup>8</sup>]]-[[Cyclooctatetraene#Cyclooctatetraenide dianion|cyclooctatetraenide]] groups are planar, as expected for a ring containing 10 [[Conjugated system|π-electrons]], and are mutually parallel, forming a [[Sandwich compound|sandwich]] containing the [[uranium]] atom. In the solid state, the rings are eclipsed, conferring ''D<sub>8h</sub>'' symmetry on the molecule. In solution the rings rotate with a low energy barrier. |
Uranocene is highly reactive toward oxygen, being [[pyrophoricity|pyrophoric]] in air but stable to [[hydrolysis]]. Considering the molecule to be U<sup>4+</sup>(C<sub>8</sub>H<sub>8</sub><sup>2−</sup>)<sub>2</sub>, the [[hapticity|η<sup>8</sup>]]-[[Cyclooctatetraene#Cyclooctatetraenide dianion|cyclooctatetraenide]] groups are planar, as expected for a ring containing 10 [[Conjugated system|π-electrons]], and are mutually parallel, forming a [[Sandwich compound|sandwich]] containing the [[uranium]] atom. In the solid state, the rings are eclipsed, conferring ''D<sub>8h</sub>'' symmetry on the molecule. In solution the rings rotate with a low energy barrier. |
||
The uranium-cyclooctatetraenyl [[chemical bond|bonding]] was shown by [[photoelectron spectroscopy]] to be primarily due to mixing of uranium 6d orbitals into ligand pi [[molecular orbitals|orbitals]] and therefore donation of electronic charge to the uranium, with a smaller such interaction involving the uranium 5f orbitals.<ref>{{cite journal |last1= Clark |first1= J. P. |last2= Green |first2= J. C. |title= An Investigation of the Electronic Structure of Bis(''eta''-cyclo-octatetraene)-actinoids by Helium-(I) and -(II) Photoelectron Spectroscopy |year= 1977 |journal= [[J. Chem. Soc., Dalton Trans.]] |issue= 5 |pages= 505–508 |doi= 10.1039/DT9770000505}}</ref> Electronic theory calculations agree with this result<ref>{{cite journal |last1= Roesch |first1= N. |last2= Streitwieser |first2= A. |title= Quasirelativistic SCF-X''alpha'' Scattered-Wave Study of Uranocene, Thorocene, and Cerocene |year= 1983 |journal= [[J. Am. Chem. Soc.]] |volume= 105 |issue= 25 |pages= 7237–7240 |doi= 10.1021/ja00363a004}}</ref> |
The uranium-cyclooctatetraenyl [[chemical bond|bonding]] was shown by [[photoelectron spectroscopy]] to be primarily due to mixing of uranium 6d orbitals into ligand pi [[molecular orbitals|orbitals]] and therefore donation of electronic charge to the uranium, with a smaller such interaction involving the uranium 5f orbitals.<ref>{{cite journal |last1= Clark |first1= J. P. |last2= Green |first2= J. C. |title= An Investigation of the Electronic Structure of Bis(''eta''-cyclo-octatetraene)-actinoids by Helium-(I) and -(II) Photoelectron Spectroscopy |year= 1977 |journal= [[J. Chem. Soc., Dalton Trans.]] |issue= 5 |pages= 505–508 |doi= 10.1039/DT9770000505}}</ref> Electronic theory calculations agree with this result<ref>{{cite journal |last1= Roesch |first1= N. |last2= Streitwieser |first2= A. |title= Quasirelativistic SCF-X''alpha'' Scattered-Wave Study of Uranocene, Thorocene, and Cerocene |year= 1983 |journal= [[J. Am. Chem. Soc.]] |volume= 105 |issue= 25 |pages= 7237–7240 |doi= 10.1021/ja00363a004}}</ref><ref name=chang><ref>{{cite journal |last1= Chang |first1= A. H. H. |last2= Pitzer |first2= R. M. |title= Electronic Structure and Spectra of Uranocene |year= 1989 |journal= [[J. Am. Chem. Soc.]] |volume= 111 |issue= 7 |pages= 2500–2507 |doi= 10.1021/ja00189a022}}</ref> and point out that the weaker interaction of the open-shell 5f orbitals with the ligand orbitals determines |M<sub>J</sub>|, the magnitude of the angular momentum quantum number along the 8-fold symmetry axis of the ground state.<ref name=chang/> |
||
===Spectroscopic properties=== |
===Spectroscopic properties=== |
||
Uranocene is [[paramagnetism|paramagnetic]]. Its [[magnetic susceptibility]] is consistent with 3 |
Uranocene is [[paramagnetism|paramagnetic]]. Its [[magnetic susceptibility]] is consistent with values of 3 or 4 for |M<sub>J</sub>|, with the accompanying magnetic moment being affected by the [[spin-orbit coupling]].<ref>{{cite journal |last1= Karraker |first1= D. G. |last2= Stone |first2= J. A. |last3= Jones |first3= E. R. |last4= Edelstein |first4= N. |title= Bis(cyclooctatetraenyl)neptunium(IV) and Bis(cyclooctatetraenyl)plutonium(IV) |year= 1970 |journal= [[J. Chem. Phys.]] |volume= 92 |issue= 16 |pages= 4841–4845 |doi= 10.1021/ja00719a014}}</ref> Its [[Nuclear magnetic resonance|NMR]] spectrum is consistent with an |M<sub>J</sub>| value of 3.<ref>{{cite book |last= Fischer |first= R. D. |editor1-last= Marks |editor1-first= T. J. |editor2-last= Fischer |editor2-first= R. D. |title= Volume 44 – Organometallics of the f-Elements |series= NATO Advanced Study Institutes Series: Series C – Mathematical and Physical Sciences |publisher= Reidel |location= Dordrecht, Holland |year= 1979 |pages= 337–377 |chapter= NMR Spectroscopy of Organometallic Compounds of the f-Elements: Practical Applications |isbn= 90-277-0990-4}}</ref> Electronic theory calculations from the simplest<ref>{{cite journal |last1= Hayes |first1= R. G. |last2= Edelstein |first2= N. |title= An Elementary Molecular Orbital Calculation on U(C<sub>8</sub>H<sub>8</sub>)<sub>2</sub> and Its Application to the Electronic Structure of U(C<sub>8</sub>H<sub>8</sub>)<sub>2</sub>, Np(C<sub>8</sub>H<sub>8</sub>)<sub>2</sub>. and Pu(C<sub>8</sub>H<sub>8</sub>)<sub>2</sub> |year= 1972 |journal= [[J. Am. Chem. Soc.]] |volume= 94 |issue= 25 |pages= 8688–8691 |doi= 10.1021/ja00780a008}}</ref> to the most accurate<ref>{{cite journal |last1= Liu |first1= W. |last2= Dolg |first2= M. |last3= Fulde |first3= P. |year= 1997 |title= Low-lying electronic states of lanthanocenes and actinocenes M(C<sub>8</sub>H<sub>8</sub>)<sub>2</sub> (M=Nd, Tb, Yb, U) |journal= [[J. Chem. Phys.]] |volume= 107 |issue= 9 |pages= 3584–3591 |doi= 10.1063/1.474698}}</ref> also give |M<sub>J</sub>| values of 3 for the ground state and 2 for the first excited state, corresponding to double-group symmetry designations<ref>{{cite book |last= Herzberg |first= G. |title= Molecular Spectra and Molecular Structure III. Electronic Spectra and Electronic Structure of Polyatomic Molecules |year= 1966 |publisher= D. Van Nostrand |location= Princeton, New Jersey |page= 566}}</ref> of E<sub>3g</sub> and E<sub>2g</sub> for these states. |
||
The green color of uranocene is due to three strong transitions in its [[visible spectrum]].<ref name=synth /><ref name=dall>{{cite journal |last1= Dallinger |first1= R. F. |last2= Stein |first2= P. |last3= Spiro |first3= T. G. |title= Resonance Raman Spectroscopy of Uranocene: Observation of an Anomalously Polarized Electronic Band and Assignment of Energy Levels |year= 1978 |journal= [[J. Am. Chem. Soc.]] |volume= 100 |issue= 25 |pages= 7865–7870 |doi= 10.1021/ja00493a013}}</ref> In addition to finding vibrational frequencies, [[Raman Spectroscopy|Raman spectra]] indicate the presence of a low-lying excited electronic state.<ref name=dall /><ref>{{cite journal |last1= Hager |first1= J. S. |last2= Zahardis |first2= J. |last3= Pagni |first3= R. M. |last4= Compton |first4= R. N. |last5= Li |first5= J. |display-authors= 3 |title= Raman under nitrogen. The high-resolution Raman spectroscopy of crystalline uranocene, thorocene, and ferrocene |year= 2004 |journal= [[J. Chem. Phys.]] |volume= 120 |issue= 6 |pages= 2708–2718 |doi= 10.1063/1.1637586 |pmid= 15268415}}</ref> On the basis of calculations the visible transitions are assigned to transitions to E<sub>2u</sub> and E<sub>3u</sub> states. |
The green color of uranocene is due to three strong transitions in its [[visible spectrum]].<ref name=synth /><ref name=dall>{{cite journal |last1= Dallinger |first1= R. F. |last2= Stein |first2= P. |last3= Spiro |first3= T. G. |title= Resonance Raman Spectroscopy of Uranocene: Observation of an Anomalously Polarized Electronic Band and Assignment of Energy Levels |year= 1978 |journal= [[J. Am. Chem. Soc.]] |volume= 100 |issue= 25 |pages= 7865–7870 |doi= 10.1021/ja00493a013}}</ref> In addition to finding vibrational frequencies, [[Raman Spectroscopy|Raman spectra]] indicate the presence of a low-lying (E<sub>2g</sub>) excited electronic state.<ref name=dall /><ref>{{cite journal |last1= Hager |first1= J. S. |last2= Zahardis |first2= J. |last3= Pagni |first3= R. M. |last4= Compton |first4= R. N. |last5= Li |first5= J. |display-authors= 3 |title= Raman under nitrogen. The high-resolution Raman spectroscopy of crystalline uranocene, thorocene, and ferrocene |year= 2004 |journal= [[J. Chem. Phys.]] |volume= 120 |issue= 6 |pages= 2708–2718 |doi= 10.1063/1.1637586 |pmid= 15268415}}</ref> On the basis of calculations,<ref name=chang/> the visible transitions are assigned to transitions to E<sub>2u</sub> and E<sub>3u</sub> states. |
||
==Analogous compounds== |
==Analogous compounds== |
||
Revision as of 15:03, 30 March 2016
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Bis(η8-cyclooctatetraenyl)uranium(IV)
| |||
| Other names
Uranium cyclooctatetraenide
U(COT)2 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C16H16U | |||
| Molar mass | 446.33 g/mol | ||
| Appearance | green crystals[1] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
ignites in air | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
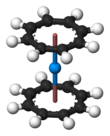
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraenide rings. It was one of the first organouranium compounds to be synthesized. It is a green air-sensitive solid that dissolves in organic solvents. Uranocene, a member of the "actinocenes," a group of metallocenes incorporating elements from the actinide series. It is the most studied bis[8]annulene-metal system, although it has no practical applications.[2]
Synthesis, structure and bonding
Uranocene was first described in 1968, when it was prepared by the reaction of dipotassium cyclooctatetraenide and uranium tetrachloride in THF at 0°C:[1]
Uranocene is highly reactive toward oxygen, being pyrophoric in air but stable to hydrolysis. Considering the molecule to be U4+(C8H82−)2, the η8-cyclooctatetraenide groups are planar, as expected for a ring containing 10 π-electrons, and are mutually parallel, forming a sandwich containing the uranium atom. In the solid state, the rings are eclipsed, conferring D8h symmetry on the molecule. In solution the rings rotate with a low energy barrier.
The uranium-cyclooctatetraenyl bonding was shown by photoelectron spectroscopy to be primarily due to mixing of uranium 6d orbitals into ligand pi orbitals and therefore donation of electronic charge to the uranium, with a smaller such interaction involving the uranium 5f orbitals.[3] Electronic theory calculations agree with this result[4]Cite error: A <ref> tag is missing the closing </ref> (see the help page). and point out that the weaker interaction of the open-shell 5f orbitals with the ligand orbitals determines |MJ|, the magnitude of the angular momentum quantum number along the 8-fold symmetry axis of the ground state.[5]
Spectroscopic properties
Uranocene is paramagnetic. Its magnetic susceptibility is consistent with values of 3 or 4 for |MJ|, with the accompanying magnetic moment being affected by the spin-orbit coupling.[6] Its NMR spectrum is consistent with an |MJ| value of 3.[7] Electronic theory calculations from the simplest[8] to the most accurate[9] also give |MJ| values of 3 for the ground state and 2 for the first excited state, corresponding to double-group symmetry designations[10] of E3g and E2g for these states.
The green color of uranocene is due to three strong transitions in its visible spectrum.[1][11] In addition to finding vibrational frequencies, Raman spectra indicate the presence of a low-lying (E2g) excited electronic state.[11][12] On the basis of calculations,[5] the visible transitions are assigned to transitions to E2u and E3u states.
Analogous compounds
Analogous compounds of the form M(C8H8)2 exist for M = (Nd, Tb, Pu, Pa, Np, Th, and Yb). Extensions include the air-stable derivative U(C8H4Ph4)2 and the cycloheptatrienyl species [U(C7H7)2]−.[2] In contrast, bis(cyclooctatetraene)iron has a very different structure, with one each of a η6- and η4-C8H8 ligands.
References
- ^ a b c Streitwieser, A.; Mueller-Westerhoff, U. (1968). "Bis(cyclooctatetraenyl)uranium (uranocene). A new class of sandwich complexes that utilize atomic f orbitals". J. Am. Chem. Soc. 90 (26): 7364–7364. doi:10.1021/ja01028a044.
- ^ a b Seyferth, D. (2004). "Uranocene. The First Member of a New Class of Organometallic Derivatives of the f Elements". Organometallics. 23 (15): 3562–3583. doi:10.1021/om0400705.
- ^ Clark, J. P.; Green, J. C. (1977). "An Investigation of the Electronic Structure of Bis(eta-cyclo-octatetraene)-actinoids by Helium-(I) and -(II) Photoelectron Spectroscopy". J. Chem. Soc., Dalton Trans. (5): 505–508. doi:10.1039/DT9770000505.
- ^ Roesch, N.; Streitwieser, A. (1983). "Quasirelativistic SCF-Xalpha Scattered-Wave Study of Uranocene, Thorocene, and Cerocene". J. Am. Chem. Soc. 105 (25): 7237–7240. doi:10.1021/ja00363a004.
- ^ a b Cite error: The named reference
changwas invoked but never defined (see the help page). - ^ Karraker, D. G.; Stone, J. A.; Jones, E. R.; Edelstein, N. (1970). "Bis(cyclooctatetraenyl)neptunium(IV) and Bis(cyclooctatetraenyl)plutonium(IV)". J. Chem. Phys. 92 (16): 4841–4845. doi:10.1021/ja00719a014.
- ^ Fischer, R. D. (1979). "NMR Spectroscopy of Organometallic Compounds of the f-Elements: Practical Applications". In Marks, T. J.; Fischer, R. D. (eds.). Volume 44 – Organometallics of the f-Elements. NATO Advanced Study Institutes Series: Series C – Mathematical and Physical Sciences. Dordrecht, Holland: Reidel. pp. 337–377. ISBN 90-277-0990-4.
- ^ Hayes, R. G.; Edelstein, N. (1972). "An Elementary Molecular Orbital Calculation on U(C8H8)2 and Its Application to the Electronic Structure of U(C8H8)2, Np(C8H8)2. and Pu(C8H8)2". J. Am. Chem. Soc. 94 (25): 8688–8691. doi:10.1021/ja00780a008.
- ^ Liu, W.; Dolg, M.; Fulde, P. (1997). "Low-lying electronic states of lanthanocenes and actinocenes M(C8H8)2 (M=Nd, Tb, Yb, U)". J. Chem. Phys. 107 (9): 3584–3591. doi:10.1063/1.474698.
- ^ Herzberg, G. (1966). Molecular Spectra and Molecular Structure III. Electronic Spectra and Electronic Structure of Polyatomic Molecules. Princeton, New Jersey: D. Van Nostrand. p. 566.
- ^ a b Dallinger, R. F.; Stein, P.; Spiro, T. G. (1978). "Resonance Raman Spectroscopy of Uranocene: Observation of an Anomalously Polarized Electronic Band and Assignment of Energy Levels". J. Am. Chem. Soc. 100 (25): 7865–7870. doi:10.1021/ja00493a013.
- ^ Hager, J. S.; Zahardis, J.; Pagni, R. M.; et al. (2004). "Raman under nitrogen. The high-resolution Raman spectroscopy of crystalline uranocene, thorocene, and ferrocene". J. Chem. Phys. 120 (6): 2708–2718. doi:10.1063/1.1637586. PMID 15268415.
Further reading
- The f elements, Nikolas Kaltsoyannis and Peter Scott. ISBN 0-19-850467-5
- Chemistry of the Elements, N. N. Greenwood and A. Earnshaw. ISBN 0-08-022057-6
- Lanthanides & Actinides: Organoactinides