Potassium phthalimide: Difference between revisions
Appearance
Content deleted Content added
Updating {{chembox}} (changes to verified and watched fields - updated 'UNII_Ref', 'Verifiedfields') per Chem/infobox_drug validation (report errors or bugs) |
m corrected |
||
| Line 72: | Line 72: | ||
| OtherCompounds = [[Phthalimide]]}} |
| OtherCompounds = [[Phthalimide]]}} |
||
}} |
}} |
||
'''Potassium phthalimide''' is a chemical compound of formula C<sub>8</sub>H<sub>4</sub>KNO<sub>2</sub>. It is commercially available, and usually presents as fluffy, very pale yellow crystals. It is the potassium salt of [[phthalimide]]. If desired, it may be prepared by adding a hot solution of phthalimide to a solution of [[potassium hydroxide]]; the desired product precipitates.<ref>{{OrgSynth | title = β-Bromoethylphthalimide | collvol = 1 | collvolpages = 119 | prep = cv1p0119 | year = 1941 | author = P. L. Salzberg and J. V. Supniewski}}</ref> |
'''Potassium phthalimide''' is a chemical compound of formula C<sub>8</sub>H<sub>4</sub>KNO<sub>2</sub>. It is commercially available, and usually presents as fluffy, very pale yellow crystals. It is the potassium salt of [[phthalimide]]. If desired, it may be prepared by adding a hot solution of phthalimide in ethanol to a solution of [[potassium hydroxide]] in ethanol; the desired product precipitates.<ref>{{OrgSynth | title = β-Bromoethylphthalimide | collvol = 1 | collvolpages = 119 | prep = cv1p0119 | year = 1941 | author = P. L. Salzberg and J. V. Supniewski}}</ref> |
||
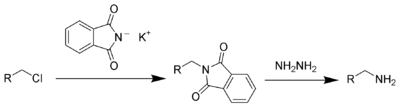
This compound is a reagent used in the [[Gabriel synthesis]] of [[amine]]s. |
This compound is a reagent used in the [[Gabriel synthesis]] of [[amine]]s. |
||
Revision as of 20:50, 11 November 2018

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.012.770 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H4KNO2 | |
| Molar mass | 185.221 g/mol |
| Appearance | Light yellow solid |
| Melting point | > 300 °C (572 °F; 573 K) |
| Soluble in water, | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Phthalimide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium phthalimide is a chemical compound of formula C8H4KNO2. It is commercially available, and usually presents as fluffy, very pale yellow crystals. It is the potassium salt of phthalimide. If desired, it may be prepared by adding a hot solution of phthalimide in ethanol to a solution of potassium hydroxide in ethanol; the desired product precipitates.[1]
This compound is a reagent used in the Gabriel synthesis of amines.
References
- ^ P. L. Salzberg and J. V. Supniewski (1941). "β-Bromoethylphthalimide". Organic Syntheses; Collected Volumes, vol. 1, p. 119.