Hydrazine sulfate
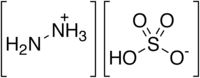
| |
| Names | |
|---|---|
| IUPAC name
Hydrazinium hydrogen sulfate
| |
| Other names
Hydrazine sulphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.088 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| H6N2O4S | |
| Molar mass | 130.12 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hydrazine sulfate is the salt of hydrazine and sulfuric acid. Known by the trade name Sehydrin, it is a chemical compound that has been used as an alternative medical treatment for the loss of appetite (anorexia) and weight loss (cachexia) which is often associated with cancer.[1][2][3][4] It has never been approved as a mainstream drug, although it is approved for use in clinical trials by the U.S. Food and Drug Administration (FDA) and is marketed in the United States as a dietary supplement.[5] It is also used in palliative care for terminal cancer patients in Russia and other countries of the former Soviet Union.[6]
The use of hydrazine sulfate as a cancer remedy was popularized by the magazine Penthouse in the mid 1990s, when Kathy Keeton, wife and business partner of the magazine's publisher Bob Guccione, used it to treat her metastatic breast cancer.[7] Keeton and other supporters of hydrazine sulfate treatment accused the U.S. National Cancer Institute (NCI) of deliberately hiding the beneficial effects of the compound, and threatened to launch a class action law suit.[8][9] The NCI denies the claims,[10] and says that there is little to no evidence that hydrazine sulfate has any beneficial effects whatsoever.[5] The position of the NCI was supported by an inquiry held by the General Accounting Office.[11]
A review of the clinical research concluded that hydrazine sulfate has never been shown to act as an anticancer agent; patients do not experience remissions or regressions of their cancer; and patients do not live longer than non-treated patients.[5][12][13]
Chemistry
Hydrazine sulfate is a commercially available form of hydrazine. It is a white solid, prepared by reacting an aqueous solution of hydrazine with sulfuric acid:[14] it is soluble in water, and the original hydrazine can be reformed by simply adjusting the pH. It has a number of laboratory uses in analytical chemistry and organic synthesis.
It may be preferred over pure hydrazine or hydrazine hydrate for laboratory use because it is easily purified if necessary (by recrystallization from water),[14] and it is less volatile and less susceptible to atmospheric oxidation on storage. These same properties make it the preferred form of hydrazine for dietary supplements and pharmaceutical trials. It is relatively inexpensive, with 100 grams of analytical grade hydrazine sulfate costing about USD20, and 100 tablets or capsules (60 milligrams hydrazine sulfate) costing USD20–60.
Background
Hydrazine sulfate was specifically developed as a result of a proposal by Joseph Gold, M.D. for a therapy that could offset the rapid loss of weight that occurs in cancer (cancer cachexia). This hypothesis was based on the fact that cancer cells are often unusually dependent on glycolysis for energy (the Warburg effect), Gold proposed that the body might offset this increased glycolysis using gluconeogenesis, which is the pathway that is the reverse of glycolysis. Since this process would require a great deal of energy, Gold thought that inhibiting gluconeogenesis might reverse this energy requirement and be an effective treatment for cancer cachexia.[15] Hydrazine is a reactive chemical that in the test tube can inactivate one of the enzymes needed for gluconeogenesis, phosphoenolpyruvate carboxykinase (PEP-CK). It was also postulated that if tumor energy gain (glycolysis) and host-energy loss (gluconeogenesis) were functionally interrelated, inhibition of gluconeogenesis at PEP CK could result in actual tumor regression in addition to reversal or arrest of cancer cachexia.[16] In this model, hydrazine sulfate is therefore thought to act by irreversibly inhibiting the enzyme phosphoenolpyruvate carboxykinase.
Clinical trials
Early Observational studies suggested that this drug might be effective and safe in about 50 percent of patients. Efficacy and safety were expressed in terms of appetite and weight improvements, performance status increase, decrease or disappearance of pain and weakness, tumor stabilization and regression and increased survival time. Other multicentric Phase II and randomized, double-blind Phase III clinical trials were carried out at the Petrov Research Institute of Oncology in St. Petersburg over a period of 17 years,[6][12] and at the Harbor-UCLA Medical Center in California over period of 10 years, respectively. Overall, the trials in California saw no statistically-significant effect on survival from hydrazine sulfate treatment, but noted increased calorie intake in these patients.[17] The authors also performed a post-hoc analysis on one or more subgroups of these patients, which they reported as suggesting a beneficial effect from treatment. The design and interpretation of this trial, and in particular the validity of this subgroup analysis, was strongly criticized in an 1990 editorial in the Journal of Clinical Oncology.[18]
Later randomized controlled trials trials failed to find any improvement in survival,[19][20] with some trials even finding decreased survival and poorer quality of life in those patients receiving hydrazine sulfate.[21] These consistently negative results have resulted in hydrazine sulfate being described as a "disproven cancer therapy" in a recent medical review.[22] Similarly, another review concluded that there was strong evidence against the use of hydrazine sulfate to treat anorexia or weight loss in cancer patients.[23]
Side effects
Hydrazine sulfate is toxic and carcinogenic,[24][25] as are many mainstream cancer treatments. Nevertheless, the side-effects reported in the various clinical trials are remarkably mild:[26] minor nausea and vomiting, dizziness and excitement, polyneuritis (inflammation of the nerves) and difficulties in fine muscle control (such as writing). However, more serious, even fatal side-effects have been reported in rare cases: one patient who developed fatal liver and kidney failure,[27] another with serious symptoms of neurotoxicity.[28] In the report of this single case of fatal liver and kidney failure, however, there was no documentation of the substance taken ("We could not obtain samples of the product ingested"), nor was there a blood test done to verify that the patient had ever taken hydrazine sulfate.[27] These side effects and other reports of hydrazine toxicity [17][18] are consistent with the hypothesis that hydrazine may play a role in the toxicity of the antibiotic isoniazid, which is thought to be metabolized to hydrazine in the body.[26]
Hydrazine sulfate is claimed to be a monoamine oxidase inhibitor (MAOI), and so to be incompatible with alcohol, tranquilizers and sleeping pills (benzodiazepines and barbiturates), and other psycho-active drugs, with pethidine (meperidine, Demerol), and with foods containing significant amounts of the amino acid tyrosine, such as most cheeses, raisins, avocados, processed and cured fish and meats, fermented products, and others.
References
- ^ Chlebowski, R. T.; Bulcavage, L.; Grosvenor, M.; et al. (1987), "Hydrazine Sulfate in Cancer Patients With Weight Loss. A Placebo-Controlled Clinical Experience", Cancer, 59 (3): 406–10, PMID 3791153
{{citation}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link). - ^ Brauer, M.; Inculet, R. I.; Bratnager, G.; Marsh, G. D.; Driedger, A. A.; Thompson, R. T. (1994), "Insulin Protects against Hepatic Bioenergetic Deterioration by Cancer Cachexia. An in-Vivo 31P Magnetic Resonance Study", Cancer Research, 54 (24): 6383–86, PMID 7987832
{{citation}}: Cite has empty unknown parameter:|1=(help)CS1 maint: multiple names: authors list (link). - ^ Chlebowski, R. T.; Bulcavage, L.; Grosvenor, M.; Oktay, E.; Block, J. B.; Chlebowski, J. S.; Ali, I.; Elashoff, R. (1990), "Hydrazine Sulfate Influence on Nutritional Status and Survival in Non-Small-Cell Lung Cancer", Journal of Clinical Oncology (8): 9–15, PMID 1688616
{{citation}}: CS1 maint: multiple names: authors list (link). - ^ Gold, J. (1999), "Long term complete response in patient with advanced, localized NSCLC with hydrazine sulfate, radiation and Carboplatin, refractory to combination chemotherapy", Proceedings of the American Association for Cancer Research (40): 642. Abstract.
- ^ a b c Questions and answers about hydrazine sulfate, National Cancer Institute, March 12, 2009
- ^ a b Filov, V. A.; Gershanovich, M. L.; Danova, L. A.; Ivin, B. A. (1995), "Experience of the Treatment with Sehydrin (Hydrazine Sulfate, HS) in the Advanced Cancer Patients", Investigational New Drugs, 13 (1): 89–97, PMID 7499115
{{citation}}: CS1 maint: multiple names: authors list (link). - ^ London, William M. (July 23, 2006), Penthouse's promotion of hydrazine sulfate
- ^ Goldberg, Burton (June 12, 2000), Holding the National Cancer Institute Accountable for Cancer Deaths
- ^ Goldberg, Burton; Trivieri, Larry; Anderson, John W., ed. (2002), Alternative medicine: the definitive guide (2nd ed.), Celestial Arts, pp. 50–51, 598, ISBN 1587611414
{{citation}}: CS1 maint: multiple names: editors list (link). - ^ Jenks, S. (1993), "Hydrazine Sulfate Ad Is "Offensive"", Journal of the National Cancer Institute, 85 (7): 528–29, doi:10.1093/jnci/85.7.528, PMID 8455198.
- ^ Nadel, M. V. (September 1995), "Cancer Drug Research—Contrary to Allegations, Hydrazine Sulfate Studies Were Not Flawed", Report to the Chairman and Ranking Minority Member, Human Resources and Intergovernmental Relations Subcommittee, House Committee on Government Reform and Oversight, Washington, D.C.: General Accounting Office, Document No. HEHS-95-141.
- ^ a b Kaegi, Elizabeth (1998), "Unconventional therapies for cancer: 4. Hydrazine sulfate. Task Force on Alternative Therapies of the Canadian Breast Cancer Research Initiative", Canadian Medical Association Journal, 158 (10): 1327–30, PMID 9614826.
- ^ Green, Saul (1997), "Hydrazine sulfate: is it an anticancer agent?", Scientific Review of Alternative Medicine, 1: 19–21
- ^ a b Adams, Roger; Brown, B. K. (1922). "Hydrazine sulfate". Organic Syntheses. 2: 37
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 1, p. 309. - ^ Gold, J. (1968), "Proposed Treatment of Cancer by Inhibition of Gluconeogenesis", Oncology, 22 (2): 185–207, PMID 5688432.
- ^ Gold, J. (1974), "Cancer Cachexia and Gluconeogenesis", Annals of the New York Academy of Sciences, 230: 103–10, PMID 4522864.
- ^ a b Chlebowski RT, Bulcavage L, Grosvenor M; et al. (1990). "Hydrazine sulfate influence on nutritional status and survival in non-small-cell lung cancer". J. Clin. Oncol. 8 (1): 9–15. PMID 1688616.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Piantadosi, S. (1990), "Hazards of small clinical trials" (PDF), Journal of Clinical Oncology, 8 (1): 1, retrieved 2009-06-03
- ^ Loprinzi CL, Goldberg RM, Su JQ; et al. (1994). "Placebo-controlled trial of hydrazine sulfate in patients with newly diagnosed non-small-cell lung cancer". J. Clin. Oncol. 12 (6): 1126–9. PMID 8201374.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Chlebowski RT, Bulcavage L, Grosvenor M; et al. (1990). "Hydrazine sulfate influence on nutritional status and survival in non-small-cell lung cancer". J. Clin. Oncol. 8 (1): 9–15. PMID 1688616.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Loprinzi CL, Kuross SA, O'Fallon JR; et al. (1994). "Randomized placebo-controlled evaluation of hydrazine sulfate in patients with advanced colorectal cancer". J. Clin. Oncol. 12 (6): 1121–5. PMID 8201373.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Vickers A (2004). "Alternative cancer cures: "unproven" or "disproven"?". CA Cancer J Clin. 54 (2): 110–8. PMID 15061600.
- ^ Yavuzsen T, Davis MP, Walsh D, LeGrand S, Lagman R (2005). "Systematic review of the treatment of cancer-associated anorexia and weight loss". J. Clin. Oncol. 23 (33): 8500–11. doi:10.1200/JCO.2005.01.8010. PMID 16293879.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hydrazine Hazard Summary, U.S. Environmental Protection Agency, January 2000.
- ^ Section 9.2.1, Environmental Health Criteria for Hydrazine, International Programme on Chemical Safety, 1987.
- ^ a b Black, M.; Hussain, H. (2000), "Hydrazine, Cancer, the Internet, Isoniazid, and the Liver" (PDF), Annals of Internal Medicine, 133 (11): 911–13, PMID 11103062
{{citation}}: CS1 maint: multiple names: authors list (link). - ^ a b Hainer, M. I.; et al. (2000), "Fatal hepatorenal failure associated with hydrazine sulfate" (PDF), Annals of Internal Medicine, 133: 877–80, PMID 11103057
{{citation}}: Explicit use of et al. in:|author=(help). - ^ Nagappan, R.; Riddell, T. (2000), "Pyridoxine therapy in a patient with severe hydrazine sulfate toxicity", Critical Care in Medicine, 28: 2116–18, PMID 10890675
{{citation}}: CS1 maint: multiple names: authors list (link).
External links
- Syracuse Cancer Research Institute
- Hydrazine sulfate / Hydrazine sulphate from the British Columbia Cancer Agency
- The Truth About Hydrazine Sulfate - Dr. Gold Speaks
- What is rocket fuel treatment? from Cancer Research UK
