Cavitation
This article's tone or style may not reflect the encyclopedic tone used on Wikipedia. (December 2007) |


Cavitation is the formation of vapour bubbles of a flowing liquid in a region where the pressure of the liquid falls below its vapor pressure. Cavitation is usually divided into two classes of behavior: inertial (or transient) cavitation, and noninertial cavitation. Inertial cavitation is the process where a void or bubble in a liquid rapidly collapses, producing a shock wave. Such cavitation often occurs in pumps, propellers, impellers, and in the vascular tissues of plants. Noninertial cavitation is the process in which a bubble in a fluid is forced to oscillate in size or shape due to some form of energy input, such as an acoustic field. Such cavitation is often employed in ultrasonic cleaning baths and can also be observed in pumps, propellers, etc.
Since the shock waves formed by cavitation are strong enough to significantly damage moving parts, cavitation is usually an undesirable phenomenon. It is specifically avoided in the design of machines such as turbines or propellers, and eliminating cavitation is a major field in the study of fluid dynamics.
Inertial cavitation
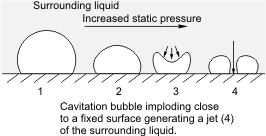
Inertial cavitation was first studied by Lord Rayleigh in the late 19th century, when he considered the collapse of a spherical void within a liquid. When a volume of liquid is subjected to a sufficiently low pressure, it may rupture and form a cavity. This phenomenon is termed cavitation inception and may occur behind the blade of a rapidly rotating propeller or on any surface vibrating underwater with sufficient amplitude and acceleration. A fast-flowing river can cause cavitation on rock surfaces, particularly when there is a drop-off, such as on a waterfall. Other ways of generating cavitation voids involve the local deposition of energy, such as an intense focused laser pulse (optic cavitation) or with an electrical discharge through a spark. Vapor gases evaporate into the cavity from the surrounding medium; thus, the cavity is not a perfect vacuum, but has a relatively low gas pressure. Such a low-pressure cavitation bubble in a liquid begins to collapse due to the higher pressure of the surrounding medium. As the bubble collapses, the pressure and temperature of the vapor within increases. The bubble eventually collapses to a minute fraction of its original size, at which point the gas within dissipates into the surrounding liquid via a rather violent mechanism, which releases a significant amount of energy in the form of an acoustic shock wave and as visible light. At the point of total collapse, the temperature of the vapor within the bubble may be several thousand kelvin, and the pressure several hundred atmospheres.
Inertial cavitation can also occur in the presence of an acoustic field. Microscopic gas bubbles that are generally present in a liquid will be forced to oscillate due to an applied acoustic field. If the acoustic intensity is sufficiently high, the bubbles will first grow in size and then rapidly collapse. Hence, inertial cavitation can occur even if the rarefaction in the liquid is insufficient for a Rayleigh like void to occur. High-power ultrasonics usually utilize the inertial cavitation of microscopic vacuum bubbles for treatment of surfaces, liquids, and slurries.
The physical process of cavitation inception is similar to boiling. The major difference between the two is the thermodynamic paths that precede the formation of the vapor. Boiling occurs when the local vapor pressure of the liquid rises above its local ambient pressure and sufficient energy is present to cause the phase change to a gas. Cavitation inception occurs when the local pressure falls sufficiently far below the saturated vapor pressure, a value given by the tensile strength of the liquid.
In order for cavitation inception to occur, the cavitation "bubbles" generally need a surface on which they can nucleate. This surface can be provided by the sides of a container, by impurities in the liquid, or by small undissolved microbubbles within the liquid. It is generally accepted that hydrophobic surfaces stabilize small bubbles. These pre-existing bubbles start to grow unbounded when they are exposed to a pressure below the threshold pressure, termed Blake's threshold.
The vapor pressure here differs from the meteorological definition of vapor pressure, which describes the partial pressure of water in the atmosphere at some value less than 100% saturation. Vapor pressure as relating to cavitation refers to the vapor pressure in equilibrium conditions and can therefore be more accurately defined as the equilibrium (or saturated) vapor pressure.
Noninertial cavitation
Noninertial cavitation is the process in which small bubbles in a liquid are forced to oscillate in the presence of an acoustic field, when the intensity of the acoustic field is insufficient to cause total bubble collapse. This form of cavitation causes significantly less erosion than inertial cavitation, and is often used for the cleaning of delicate materials, such as silicon wafers.
Cavitation damage

Cavitation is, in many cases, an undesirable occurrence. In devices such as propellers and pumps, cavitation causes a great deal of noise, damage to components, vibrations, and a loss of efficiency.
When the cavitation bubbles collapse, they force liquid energy into very small volumes, thereby creating spots of high temperature and emitting shock waves, the latter of which are a source of noise. The noise created by cavitation is a particular problem for military submarines, as it increases the chances of being detected by passive sonar.
Although the collapse of a cavity is a relatively low-energy event, highly localized collapses can erode metals, such as steel, over time. The pitting caused by the collapse of cavities produces great wear on components and can dramatically shorten a propeller or pump's lifetime.
After a surface is initially affected by cavitation, it tends to erode at an accelerating pace. The cavitation pits increase the turbulence of the fluid flow and create crevasses that act as nucleation sites for additional cavitation bubbles. The pits also increase the components' surface area and leave behind residual stresses. This makes the surface more prone to stress corrosion.[1]
Hydrodynamic Cavitation
Hydrodynamic cavitation describes the process of vaporisation, bubble generation and bubble implosion which occurs in a flowing liquid as a result of a decrease and subsequent increase in pressure. Cavitation will only occur if the pressure declines to some point below the saturated vapor pressure of the liquid. In pipe systems, cavitation typically occurs either as the result of an increase in the kinetic energy (through an area constriction) or an increase in the pipe elevation.
Hydrodynamic cavitation can be produced by passing a liquid through a constricted channel at a specific velocity or by mechanical rotation through a liquid. In the case of the constricted channel and based on the specific (or unique) geometry of the system, the combination of pressure and kinetic energy can be created when the hydrodynamic cavitation cavern downstream of the local constriction generating high energy cavitation bubbles.
The process of bubble generation, subsequent growth and collapse of the cavitation bubbles results in very high energy densities, resulting in very high temperatures and pressures at the surface of the bubbles for a very short time. The overall liquid medium environment, therefore, remains at ambient conditions. When uncontrolled, cavitation is damaging; however, by controlling the flow of the cavitation the power is harnessed and non-destructive. Controlled cavitation can be used to enhance chemical reactions or propagate certain unexpected reactions because free radicals are generated in the process due to disassociation of vapors trapped in the cavitating bubbles.
Chemical engineering applications
In industry, cavitation is often used to homogenize, or mix and break down, suspended particles in a colloidal liquid compound such as paint mixtures or milk. Many industrial mixing machines are based upon this design principle. It is usually achieved through impeller design or by forcing the mixture through an annular opening that has a narrow entrance orifice with a much larger exit orifice. In the latter case, the drastic decrease in pressure as the liquid accelerates into a larger volume induces cavitation. This method can be controlled with hydraulic devices that control inlet orifice size, allowing for dynamic adjustment during the process, or modification for different substances. The outer surface of this type of mixing valve, upon which the cavitation bubbles are driven against to cause their implosion, undergoes tremendous stress, and is often constructed of super-hard or tough materials such as stainless steel, Stellite, or even polycrystalline diamond (PCD).
Cavitating water purification devices have also been designed, in which the extreme conditions of cavitation can break down pollutants and organic molecules. Spectral analysis of light emitted in sonochemical reactions reveal chemical and plasma-based mechanisms of energy transfer. The light emitted from cavitation bubbles is termed sonoluminesence.
Hydrophobic chemicals are attracted underwater by cavitation as the pressure difference between the bubbles and the liquid water forces them to join together. This effect may assist in protein folding.[2]
Biomedical application
Cavitation plays an important role for the destruction of kidney stones in shock wave lithotripsy. Currently, tests are being conducted as to whether cavitation can be used to transfer large molecules into biological cells (sonoporation). Nitrogen cavitation is a method used in research to lyse cell membranes while leaving organelles intact. Cavitation plays a key role in non-thermal noninvasive fractionation of tissue for treatment of a variety of diseases.[3] Cavitation also probably plays a role in HIFU, a thermal noninvasive treatment methodology for cancer.[4]
Cleaning application
In industrial cleaning applications, cavitation has sufficient power to overcome the particle-to-substrate adhesion forces, loosening contaminants. The threshold pressure required to initiate cavitation is a strong function of the pulse width and the power input. This method works by generating controlled acoustic cavitation in the cleaning fluid, picking up and carrying contaminant particles away so that they do not reattach to the material being cleaned.
Pumps and propellers
Major places where cavitation occurs are in pumps, on propellers, or at restrictions in a flowing liquid.
As an impeller's (in a pump) or propeller's (as in the case of a ship or submarine) blades move through a fluid, low-pressure areas are formed as the fluid accelerates around and moves past the blades. The faster the blades move, the lower the pressure around it can become. As it reaches vapor pressure, the fluid vaporizes and forms small bubbles of gas. This is cavitation. When the bubbles collapse later, they typically cause very strong local shock waves in the fluid, which may be audible and may even damage the blades.
Cavitation in pumps may occur in two different forms:
Suction cavitation
Suction cavitation occurs when the pump suction is under a low-pressure/high-vacuum condition where the liquid turns into a vapor at the eye of the pump impeller. This vapor is carried over to the discharge side of the pump, where it no longer sees vacuum and is compressed back into a liquid by the discharge pressure. This imploding action occurs violently and attacks the face of the impeller. An impeller that has been operating under a suction cavitation condition can have large chunks of material removed from its face or very small bits of material removed, causing the impeller to look spongelike. Both cases will cause premature failure of the pump, often due to bearing failure. Suction cavitation is often identified by a sound like gravel or marbles in the pump casing.
Discharge cavitation
Discharge cavitation occurs when the pump discharge pressure is extremely high, normally occurring in a pump that is running at less than 10% of its best efficiency point. The high discharge pressure causes the majority of the fluid to circulate inside the pump instead of being allowed to flow out the discharge. As the liquid flows around the impeller, it must pass through the small clearance between the impeller and the pump housing at extremely high velocity. This velocity causes a vacuum to develop at the housing wall (similar to what occurs in a venturi), which turns the liquid into a vapor. A pump that has been operating under these conditions shows premature wear of the impeller vane tips and the pump housing. In addition, due to the high pressure conditions, premature failure of the pump's mechanical seal and bearings can be expected. Under extreme conditions, this can break the impeller shaft.
Discharge cavitation in joint fluid is thought to cause the popping sound produced by bone joint cracking, for example by deliberately cracking one's knuckles.
Cavitation in engines
Some bigger diesel engines suffer from cavitation due to high compression and undersized cylinder walls. Vibrations of the cylinder wall induce alternating low and high pressure in the coolant against the cylinder wall. The result is pitting of the cylinder wall, which will eventually let cooling fluid leak into the cylinder and combustion gases to leak into the coolant.
It is possible to prevent this from happening with the use of chemical additives in the cooling fluid that form a protective layer on the cylinder wall. This layer will be exposed to the same cavitation, but rebuilds itself.
From about the 1980s, new designs of smaller petrol engines also displayed cavitation phenomenon. One answer to the need for smaller and lighter engines was a smaller coolant volume and a correspondingly higher coolant velocity. This gave rise to rapid changes in flow velocity and therefore rapid changes of static pressure in areas of high heat transfer. Where resulting vapour bubbles collapsed against a surface, they had the effect of first disrupting protective oxide layers (of cast aluminum materials) and then repeatedly damaging the newly formed surface, preventing the action of some types of corrosion inhibitor (such as silicate based inhibitors). A final problem was the affect that increased material temperature had on the relative electrochemical reactivity of the base metal and its alloying constituents. The result was deep pits that could form and penetrate the engine head in a matter of hours when the engine was running at high load and high speed. These effects could largely be avoided by the use of organic corrosion inhibitors or (preferably) by designing the engine head in such a way as to avoid certain cavitation inducing conditions.
Vascular plants
Cavitation occurs in the xylem of vascular plants when the tension of water within the xylem becomes so great that dissolved air within the water expands to fill either the vessel elements or tracheids. Plants are generally able to repair cavitated xylem in a number of ways. For plants less than 50 cm tall, root pressure can be sufficient to redissolve air. For larger plants, they must repair cavitation by importing solutes into the xylem; this causes water to enter as well, which can then redissolve the air. In some trees, the sound of the cavitation is clearly audible, particularly in summer, when the rate of evapotranspiration is highest. Deciduous trees shed leaves in the autumn partly because cavitation increases as temperatures decrease.
Marine life
Just as cavitation bubbles form on a fast-spinning boat propeller, they may also form on the tails and fins of aquatic animals. The effects of cavitation are especially important near the surface of the ocean, where the ambient water pressure is relatively low and cavitation is more likely to occur.
For powerful swimming animals like dolphins and tuna, cavitation may be detrimental, because it limits their maximum swimming speed.[5] Even if they have the power to swim faster, dolphins may have to restrict their speed because collapsing cavitation bubbles on their tail are too painful. Cavitation also slows tuna, but for a different reason. Unlike dolphins, these fish do not feel the painful bubbles, because they have bony fins without nerve endings. Nevertheless, they cannot swim faster because the cavitation bubbles create an air film around their fins that limits their speed. Lesions have been found on tuna that are consistent with cavitation damage.
Cavitation is not always a limitation for sea life; some animals have found ways to use it to their advantage when hunting prey. The pistol shrimp snaps a specialized claw to create cavitation, which can kill small fish. The mantis shrimp (of the smasher variety) uses cavitation as well in order to stun, smash open, or kill the shellfish that it feasts upon.
Coastal erosion
In the last half-decade, coastal erosion in the form of inertial cavitation has been generally accepted.[6] Air pockets in an incoming wave are forced into cracks in the cliff being eroded, then the force of the wave compresses the air pockets until the bubble implodes, becoming liquid, giving off various forms of energy that blast apart the rock.
List of cavitation tunnels
Canada
- National Research Council—Institute for Ocean Technology Cavitation Tunnel,[7] St. Johns, Newfoundland.
France
- "Tunnel de Cavitation" Ecole Navale,[8] Lanveoc.
- "Grand Tunnel Hydrodynamique" Bassin d'Essais des Carènes,[9] Val de Reuil.
Germany
- Multiple cavitation tunnels at the Versuchsanstalt für Wasserbau und Schiffbau,[10] Berlin.
- Large Cavitation tunnel at Hamburg Ship Model Basin,[11] Hamburg.
India
- Fluid Control Research Institute, Palakkad,Kerala. http://www.fcriindia.com
- Cavitation Tunnel of the Naval Science and Technology Labs at Visakhapatnam.
Iran
- Applied Hydrodynamics Laboratory, Iran University of Science and Technology,[12] Narmak, Tehran.
- Marine Engineering Laboratory, Sharif University of Technology,[13][14] Azadi Av., Tehran.
Netherlands
- Large Cavitation Tunnel and High Speed Cavitation Tunnel[15] at the Maritime Research Institute, Wageningen.
Norway
- "Cavitation Lab" NTNU, The Norwegian University of Science and Technology,[16] Trondheim.
Poland
- Ship Design and Research Centre (CTO S.A.) Centrum Techniki Okrętowej S.A.,[17] Gdansk.
South Korea
- Samsung Ship Model Basin (SSMB), Samsung Heavy Industries,[18] Daejeon.
Spain
- CEHIPAR (Canal de Experiencias Hidrodinámicas de El Pardo),[19] El Pardo (Madrid).
Sweden
- SSPA[20]
Taiwan
- The Large Cavitation Tunnel at National Taiwan Ocean University, Keelung.
United Kingdom
United States
- The Garfield Thomas Water Tunnel, The Pennsylvania State University,[22] State College, PA.
- The William B. Morgan Large Cavitation Channel,[23] Memphis, TN.
- MIT's variable pressure water tunnel.[24]
- University of Minnesota's St. Anthony Falls Laboratory cavitation facilities.
See also
- The phenomenon known as supercavitation is used to allow objects to travel under water at high speed.
- Supercavitation propeller
- Sonoluminescence
- Cavitation number
- Ultrasonics
- Erosion corrosion of copper water tubes
- Water hammer
- Mitton Valve
References
- ^ Stachowiak, G.W.; Batchelor, A.W. (2001). Engineering tribology. Boston: Butterworth-Heinemann. p. 525. ISBN 0750673044.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help)CS1 maint: multiple names: authors list (link) - ^ "Sandia researchers solve mystery of attractive surfaces". Sandia National Laboratories. 2006-08-02. Retrieved 2007-10-17.
{{cite web}}: Check date values in:|date=(help) - ^ University of Michigan. Therapeutic Ultrasound Group, Biomedical Engineering Department, University of Michigan.
- ^ University of Oxford. Biomedical Ultrasonics & Biotherapy Laboratory, Institute of Biomedical Engineering, University of Oxford.
- ^ Brahic, Catherine (2008-03-28). "Dolphins swim so fast it hurts". NewScientist. Retrieved 2008-03-31.
- ^ Panizza, Mario (1996). Environmental Geomorphology. Amsterdam; New York: Elsevier. pp. 112–115. ISBN 0444898301.
- ^ Cavitation Tunnel - NRC-IOT
- ^ Ecole Navale - Ecole Navale
- ^ cadre
- ^ VWS-Berlin
- ^ HSVA - Hamburgische Schiffbau-Versuchsanstalt
- ^ http://hydrolab.iust.ac.ir
- ^ http://mech.sharif.edu/~mel/
- ^ http://mech.sharif.edu/~mel/CAVITATIONAL%20TUNEL.html
- ^ http://www.marin.nl/web/show/id=45465%7C
- ^ Cavitation lab | IMT, IVT- NTNU
- ^ Cavitation tunnel
- ^ http://www.shi.samsung.co.kr/eng/
- ^ Canal de Experiencias Hidrodinámicas de El Pardo
- ^ SSPA provides you with efficient maritime solutions! | SSPA Sweden
- ^ Emerson Cavitation Tunnel
- ^ GTWT
- ^ http://www50.dt.navy.mil/facilities/LCC.html
- ^ MIT Marine Hydrodynamics Laboratory
- S. Barnett; Nonthermal issues: Cavitation—Its nature, detection and measurement; Ultrasound in Medicine & Biology, Volume 24, Supplement 1, June 1998, Pages S11-S21
Further reading
For cavitation in plants, see Plant Physiology by Taiz and Zeiger. For cavitation in the engineering field, visit [1]
- Kornfelt, M.: "On the destructive action of cavitation," Journal of applied Physics No.15, 1944.
