Area postrema
| Area postrema | |
|---|---|
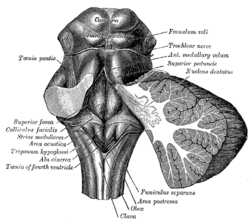 Rhomboid fossa. (Area postrema labeled at bottom center.) | |
| Identifiers | |
| Acronym(s) | AP |
| MeSH | D031608 |
| NeuroNames | 772 |
| NeuroLex ID | birnlex_2636 |
| TA98 | A14.1.04.258 |
| TA2 | 6009 |
| FMA | 72607 |
| Anatomical terms of neuroanatomy | |
The area postrema is a part of the brain that controls vomiting.
Anatomy
The area postrema is a small protuberance found at the inferoposterior limit of the fourth ventricle. Specialized ependymal cells are found within the area postrema. These specialized ependymal cells differ slightly from the majority of ependymal cells (ependymocytes) forming a unicellular epithelium lining of the ventricles and central canal. The area postrema is separated from the vagal triangle by the funiculus separans, a thin semitransparent ridge. The vagal triangle overlies the dorsal vagal nucleus and it situated on the caudal end of the rhomboid fossa or 'floor' of the fourth ventricle. The area postrema is situated just before the obex, the inferior apex of the caudal ventricular floor. Both the funiculus separans and area postrema have a similar thick ependyma-containing tanycyte covering. Ependyma and tanycytes can participate in transport of neurochemicals into and out of the cerebrospinal fluid from its cells or adjacent neurons, glia or vessels. Epednyma and tanycytes may also participate in chemoreception. The eminence of the area postrema is considered a circumventricular organ because its endothelial cells do not contain tight junctions, which allows for free exchange of molecules between blood and brain tissue. This unique breakdown in the blood-brain barrier is partially compensated for by the presence of a tanycyte barrier. [1] Recent research investigating of rat neurology has articulated more specific neural connections between the area postrema and its surrounding central structures. These connections, when considered in the context of the known vagal afferent input and reduced blood-brain barrier of AP, place this structure in a unique position to receive and modulate ascending interoceptive information and to influence autonomic outflow as well. [2]
Function
The area postrema, one of the circumventricular organs,[3] detects toxins in the blood and acts as a vomit inducing center. It connects to the nucleus of the solitary tract and other autonomic control centers in the brainstem. It is thus excited by visceral afferent impulses (sympathetic and vagal) arising from the gastrointestinal tract and other peripheral trigger areas.
The area postrema is a critical homeostatic integration center for humoral and neural signals. Recent studies have implicated its function as a chemoreceptor trigger site for vomiting in response to emetic drugs. The area postrema makes up part of the dorsal vagal complex, which is the critical termination site of vagal afferent nerve fibers, along with the dorsal motor nucleus of the vagus and the nucleus of the solitary tract. Vomiting and nausea are most likely induced by the area postrema through its connection to the nucleus of the solitary tract, which may serve as the beginning of the pathway triggering vomiting in response to various emetic inputs. However, this structure plays no key role for vomiting induced by the activation of vagal nerve fibers or by motion, and its function in radiation-induced vomiting remains unclear.
The area postrema’s position outside of the blood-brain barrier makes this particular region of the medulla a key player in the autonomic control of various physiological systems, including the systems controlling feeding and metabolism and cardiovascular system. It is a densely vacularized structure which lacks tight junctions between endothelial cells, thereby allowing it to detect various toxins in the blood as well as in the cerebrospinal fluid. Because the area postrema is located outside of the blood-brain barrier, peptide and other physiological signals in the blood have direct access to neurons of brain areas with vital roles in the autonomic control of the body. As a result, the area postrema is now being considered as the initial site for integration for various physiological signals in the blood as they enter the central nervous system.
History of research and Current research
The area postrema was first named and located in the gross anatomy of the brain by Magnus Gustaf Retzius, a Swedish anatomist, anthropologist and professor of histology at the Karolinska Mediko-Kirurgiska Institutet in Stockholm.[4] In 1896 he published a two-volume monograph on the gross anatomy of human brain in which the area postrema was mentioned. This work was one of the most important works published in the 19th century on the anatomy of the human brain.[5]
In 1937, a publication by King, L.S. claimed that the area postrema was made up soley of glial cells, but this was later disproved by the research of several scientists including Jan Cammermeyer, Kenneth R. Brizzee and Herbert L. Borison who demonstrated the presence of neurons in the area postrema of several mammal species.[6]
Scientists became increasingly interested in the research of of vomiting in the 1950s, perhaps in part due to society's heightened awareness of radiation sickness, a condition in which many patients who vomited after radiation exposure died. Intensive studies on vomiting began in the 1950s at the University of Utah College of Medicine where Borison held a strong presence as both a professor and a researcher.[7]. He had received his doctorate in 1948 from Colombia University, establishing himself as an authority on brainstem and neurophysiology.[8] Prior to the research of Borison and his well-known colleague S.C. Wang, a doctor and assistant professor from Columbia University, it was believed that the human body's chemodetection and coordination of vomiting, or emesis, were controlled exclusively by the dorsal vagal nucleus. Yet this idea was "incompatible with the observation that emesis could still be induced by gastrointestinal irritants in dogs with chronic lesions of the dorsal vagal nucleus", and so Borison and Wang dedicated their research to solving this puzzle. Borison eventually explained that their results showed the existence of two areas in the brain related to emesis; one, a chemosensor for vomiting with no coordinating function, located in the fourth ventricle and two, a coordinator of vomiting with no chemosensory function, located in the lateral reticular formation of the medulla oblongata.[9]
In 1953 Borison and Wang determined that the chemosensor area acted as a vomiting trigger zone in the brain stem and they named it the chemoreceptor trigger zone (CTZ) for emesis. Using cats and dogs as model organisms, they found that the removal of this trigger zone from the brain allowed for the prevention of emesis in the animals directly following injection of certain chemicals into the blood stream, demonstrating the existence of a relationship between the trigger zone and the act of vomiting.[10]. The CTZ was anatomically located in the area postrema of the medulla oblongata. The area postrema had been anatomically identified and named nearly 60 years earlier, but its function had remained unknown until the work of Borison and Wang proposed its role in emesis, which was later confirmed by many laboratories.[11]
Borison and Wang created the Borison-Wang model of emesis which includes a diagram illustrating their concept of a vestibular emetic pathway connecting through the area postrema as the obligatory route to the emetic center, however, more recent research has shown this model to be incorrect.[12]
Other scientists noted as pioneers in the field of research concerning the area postrema and the mechanism of vomiting in general are Larry McCarthy, A.D. Miller and V. J Wilson.
Research has continued today around the world on the functions of the area postrema. Beyond its role in emesis, as studied intensely by the researchers of the mid 1900s, the activity of the area postrema has been closely linked to other autonomic functions such as regulation of food intake, body fluid homeostasis and cardiovascular regulation through behavioral studies and electrophysiological studies. In 2007 in Japan, research was performed on the mechanism of excitability of area postrema neurons by extracellular ATP. Voltage clamp whole-cell recording techniques were used on rat brain slices. The results showed that most responses to ATP were excitatory and that they were mediated by particular P2 purinoceptors found in the area postrema.[13] The role of the area postrema in flavor conditioned aversion and preference was studied in 2001 by researchers at the Brooklyn College at the City University of New York. The experiment tested the effect of area postrema lesions in rats on their ability to learn to flavor conditioned aversion to flavors paired with toxic drug treatments, which indeed showed that lesions of the area postrema led to impaired flavor aversion learning.
References
- ^ Gray’s anatomy : the anatomical basis of medicine and surgery (38th ed.). New York: Churchill Livingstone. 1995. ISBN 0443045607.
{{cite book}}: Unknown parameter|editorn-first=ignored (help); Unknown parameter|editorn-last=ignored (help) - ^ Shapiro, Robert E. (1985). "The central neural connections of the area postrema of the rat". The Journal of Comparative Neurology. 234 (3). Copyright © 1985 Alan R. Liss, Inc.: 344–364. doi:10.1002/cne.902340306.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ganong WF (2000). "Circumventricular organs: definition and role in the regulation of endocrine and autonomic function". Clin. Exp. Pharmacol. Physiol. 27 (5–6): 422–7. PMID 10831247.
- ^ “incremental lines of Retzius.” Merriam-Webster’s Medical Dictionary. 2009. Merriam-Webster Online. 25 Oct. 2009 <http://www.merriam-webster.com/medical/incremental%20lines%20of%20retzius>.
- ^ "Magnus Gustaf Retzius." Encyclopædia Britannica. 2009. Encyclopædia Britannica Online. 25 Oct. 2009 <http://www.britannica.com/EBchecked/topic/500204/Magnus-Gustaf-Retzius>.
- ^ Brizzee, Kenneth R., and P. M. Klara. "The ultrastructural morphology of the squirrel monkey area postrema." Cell and Tissue Research 160.3 (1975): 315-26. SpringerLink. Springer Berlin / Heidelberg, 24 Nov. 2004. Web. <http://www.springerlink.com/content/l16n2646h115k727/>.
- ^ "Medicine: Radiation Mystery." Time, 23 Jan. 1956. Web. <http://www.time.com/time/magazine/article/0,9171,861927,00.html>.
- ^ "Herbert L. Borison; Pharmacologist, 68." Obituaries. NY Times, 12 Dec. 1990. Web. <http://www.nytimes.com/1990/12/12/obituaries/herbert-l-borison-pharmacologist-68.html>.
- ^ Bianchi, Armand L. Mechanisms and control of emesis: a satellite symposium of the European Neuroscience Association : proceedings of an international meeting held in Marseille. Vol. 223. John Libbey Eurotext, 1992. Google books. Web. <http://books.google.com/books?id=vb3G_22QLI0C&source=gbs_navlinks_s>.
- ^ "Medicine: Radiation Mystery." Time, 23 Jan. 1956. Web. <http://www.time.com/time/magazine/article/0,9171,861927,00.html>.
- ^ Miller, Alan D., David J. Stewart, and John Kucharczyk. Nausea and Vomiting: Recent Research and Clinical Advances. Boca Raton: CRC, 1991. Google books. Web. <http://books.google.com/books?id=eVt0fvbJKBEC&dq=herbert+borison&source=gbs_navlinks_s>.
- ^ Grant, Virginia L., and Herbert L. Borison. "Comments on the Borison-Wang model of emesis." Psychopharmacology 96.2 (1988). SpringerLink. Web. <http://springerlink.com/content/w831718814383mj6/?p=0e9e7af3e2c94186be6c943ab3c8399e&pi=0>.
- ^ Kodama, Naoki, Makoto Funahashi, Yoshihiro Mitoh, Shogo Minagi, and Ryuji Matsuo. "Purinergic modulation of area postrema neuronal excitability in rat brain slices." Brain Research 1165 (2007): 50-59. ScienceDirect. Web. 26 Oct. 2009. <http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6SYR-4P00848-4&_user=521319&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_searchStrId=1063610022&_rerunOrigin=google&_acct=C000026018&_version=1&_urlVersion=0&_userid=521319&md5=643ef8eb4bcfd9204b90a19ba18ba51d>.
Cite error: The opening <ref> tag is malformed or has a bad name (see the help page).
