Wet sulfuric acid process
The wet sulfuric acid process (WSA process) is one out of many gas desulfurization processes on the market today. Since its introduction in the 1980s, where it was patented by the Danish catalyst company Haldor Topsoe, it has been recognised as an efficient process for recovering sulfur from various process gasses in the form of commercial quality sulfuric acid (H2SO4). The WSA process is applied in all industries where removal of sulfur is an issue.
The wet catalysis process is especially suited for processing one or more sulfur containing streams such as.[1]:
- H2S gas from e.g. amine gas treating unit
- Off-gas from Sour Water Stripper (SWS gas)
- Off-gas from Rectisol
- Spent acid from e.g. Alkylation
- Claus process tail gas
- Heavy residue or petcoke-fired utility boiler off-gas
- Boiler flue gases from various processes
- Metallurgical process gas
Another purpose for this technology is: Spent acid regeneration and production of sulfuric acid.
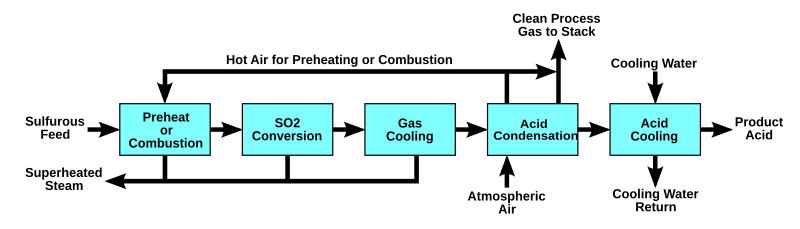
The WSA process can also be used for production of sulfuric acid from sulfur burning or for regenerating the spent acid from e.g. alkylation units. Wet catalysis processes differ from other contact sulfuric acid processes in that the feed gas contains excess moisture when it comes into contact with the catalyst. The sulfur trioxide formed by catalytic oxidation of the sulfur dioxide reacts instantly with the moisture to produce sulfuric acid in the vapour phase to an extent determined by the temperature. Liquid acid is subsequently formed by condensation of the sulfuric acid vapour and not by absorption of the sulfur trioxide in concentrated sulfuric acid, as is the case in contact processes based on dry gases.
The concentration of the product acid depends on the H2O/SO3 ratio in the catalytically converted gases and on the condensation temperature.[2] Cite error: The <ref> tag has too many names (see the help page).
The combustion gasses are cooled to the converter inlet temperature of about 420-440 °C. To process these wet gasses in a conventional cold-gas contact process (DCDA) plant would necessitate cooling and drying of the gas to remove all moisture. Therefore in many cases WSA processes is a more cost-efficient way of producing sulfuric acid.
The Process
The main reactions in the WSA process
- Combustion: H2S + 1.5 O2 = H2O + SO2 + 518 kJ/mole
- Oxidation: SO2 + ½O2 = SO3 + 99 kJ/mole (in the presence of a vanadium (V) oxide catalyst)
- Hydration: SO3 + H2O = H2SO4 (g) + 101 kJ/mole
- Condensation: H2SO4 (g) = H2SO4 (l) + 90 kJ/mole
The energy produced by these above mentioned reactions is used for steam production.
Approximately 2-3 ton high pressure steam / ton acid produced
Applications
Industries where WSA process units are installed:
- Refinery and petrochemical industry
- Metallurgy industry
- Coal-based industry (coking and gasification)
- Power industry
- Viscose industry
- Sulphuric acid industry
About 80% to 85% of the world’s sulfur production is used to manufacture sulfuric acid. Half of the world’s sulfuric acid production is used in fertilizer production, mainly to convert phosphates to water-soluble forms, according to the Fertilizer Manual, published jointly by the United Nations Industrial Development Organization (UNIDO) and IFDC.
[3]
References
- ^ Gary, J.H. and Handwerk, G.E. (1984). Petroleum Refining Technology and Economics (2nd Edition ed.). Marcel Dekker, Inc. ISBN 0824771508.
{{cite book}}:|edition=has extra text (help)CS1 maint: multiple names: authors list (link) - ^ Sulphur recovery; (2007). The Process Principles in sulphur recovery by the WSA process.). Denmark: Jens Kristen Laursen, Haldor Topsoe A/S. Reprinted from Hydrocarbonengineering August 2007
- ^ [1]; (July 2008). IFDC FOCUS ON FERTILIZERS AND FOOD SECURITY,Issue 4; Global Shortage of Sulfuric Acid Contributes to Rising Fertilizer Costs