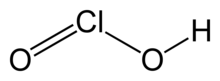
Chlorous acid
| |

| |
| Names | |
|---|---|
| IUPAC name
Chlorous acid
| |
| Identifiers | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| HClO2 | |
| Molar mass | 68.46 g/mol |
| Acidity (pKa) | 1.96 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chlorous acid is a chemical compound with the formula HClO2. It is a weak acid. Chlorine possesses oxidation state +3 in this acid. The pure substance is unstable, disproportionating to hypochlorous acid (Cl oxidation state +1) and chloric acid (Cl oxidation state +5).
2HClO2(aq) → HClO(aq) + HClO3(aq)
Chlorite salts such as sodium chlorite are stable conjugate bases derived from this acid. These salts are sometimes used in the production of chlorine dioxide.
Preparation
HClO2 can be prepared through reaction of barium chlorite and dilute sulfuric acid:
- Ba(ClO2)2 + H2SO4 → BaSO4 + 2HClO2
Stability
Chlorine is the only one of the four halogens to form an isolable acid of formula HXO2.[1] Fluorine does not have the bonding capacity to do so, whereas the hypothetical bromous acid and iodous acid have never been isolated and only a few salts of bromous acid, bromites, are known, and no iodites.[1]
References
