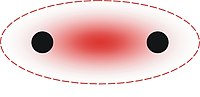
Pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds where two lobes of one involved electron orbital overlap two lobes of the other involved electron orbital. Only one of the orbital's nodal planes passes through both of the involved nuclei.

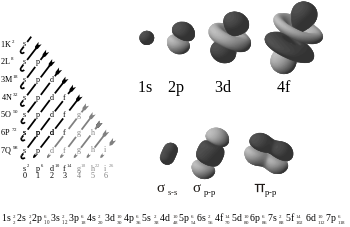
The Greek letter π in their name refers to p orbitals, since the orbital symmetry of the pi bond is the same as that of the p orbital when seen down the bond axis. P orbitals usually engage in this sort of bonding. D orbitals are also assumed to engage in pi bonding but this is not necessarily the case in reality, although the concept of bonding d orbitals still accounts well for hypervalence.
Pi bonds are usually weaker than sigma bonds. From the perspective of quantum mechanics, this bond's weakness is explained by significantly less overlap between the component p-orbitals due to their parallel orientation.
Pi bonds result from overlap of atomic orbitals that are in contact through two areas of overlap. Pi-bonds are more diffuse bonds than the sigma bonds. Electrons in pi bonds are sometimes referred to as pi electrons. Molecular fragments joined by a pi bond cannot rotate about that bond without breaking the pi bond, because rotation involves destroying the parallel orientation of the constituent p orbitals.
Multiple bonds
Atoms connected via a double bond or triple bond have, in addition to one sigma bond, one or two pi bonds, respectively.
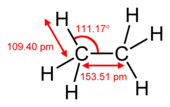
A pi bond is weaker than a sigma bond, but the combination of pi and sigma bond is stronger than either bond by itself. The enhanced strength of a multiple bond versus a single (sigma bond) is indicated in many ways, but most obviously by a contraction in bond lengths. For example in organic chemistry, carbon-carbon bond lengths are in ethane (154 pm), in ethylene (134 pm) and in acetylene (120 pm). More bonds make the total bond shorter and stronger.
 |
 |
 |
| ethane | ethylene | acetylene |
Special cases
Pi bonds do not necessarily connect a pair of atoms that are also sigma-bonded.
In certain metal complexes, pi interactions between a metal atom and alkyne and alkene pi antibonding orbitals form pi-bonds.
In some cases of multiple bonds between two atoms, there is no sigma bond at all, only pi bonds. Examples include diiron hexacarbonyl (Fe2(CO)6), dicarbon (C2) and the borane B2H2. In these compounds the central bond consists only of pi bonding, and in order to achieve maximum orbital overlap the bond distances are much shorter than expected.[1]
See also
References
- ^ Bond length and bond multiplicity: σ-bond prevents short π-bonds Eluvathingal D. Jemmis, Biswarup Pathak, R. Bruce King, Henry F. Schaefer III Chemical Communications, 2006, 2164 - 2166 Abstract