Polyacetylene
| Names | |
|---|---|
| IUPAC name
Polyethyne
| |
| Other names
Polyacetylene, PAc
| |
| Identifiers | |
| ChemSpider | |
| Properties | |
| [C2H2]n | |
| insoluble | |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Polyacetylene (IUPAC name: polyethyne) is an organic polymer with the repeating unit (C2H2)n. The high electrical conductivity discovered for these polymers beginning in the 1960s accelerated interest in the use of organic compounds in microelectronics (organic electronics). In some polyacetylenes H atoms are replaced with alkyl groups.
Structure of polyacetylene
The polymer consists of a long chain of carbon atoms with alternating single and double bonds between them, each with one hydrogen atom. Schematically the structure of polyacetylene is shown below.

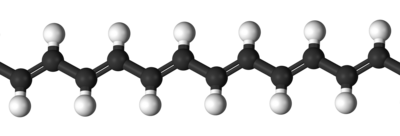
One distinguishes trans-polyacetylene, with all double bonds in the trans configuration, from cis-polyactylene, with all double bonds in the cis configuration. Each hydrogen atom can be replaced by a functional group.
Preparation
Acetylene polymerizes in a similar fashion to ethylene: the polymerization can be effected with anionic, cationic, and radical initiators. Polyacetylene is generally not prepared by polymerizing acetylene, which is a highly flammable gas that uncontrollably oligomerizes at high concentrations. The most common syntheses use ring opening metathesis polymerisation ("ROMP") of molecules like cyclooctatetraene and substituted derivatives thereof.[1][2][3]
Conductivity and the Nobel Prize
The 1964 monograph Organic Semiconductors,[4] references several previous reports of high-conductivity oxidized polyacetylenes. Similarly, highly-conductive organic charge-transfer complexes were reported by several groups in the 1950s (see conductive polymer and organic conductor).
Interest in the conductive properties of oxidatively doped polyacetylenes was reignited in the mid 1970s with the accidental discovery of a silvery, conductive polyacetylene by the research group of Professor Hideki Shirakawa. The student had polymerized acetylene with 1000 times the amount of catalyst normally used when performing the reaction. Shirakawa later collaborated with physicist Alan J. Heeger and chemist Alan G MacDiarmid, discovering in 1976 that oxidation of this material with iodine results in a 108-fold increase in conductivity. The conductivity of this doped material can approach the conductivity of the best available conductor, silver. The three were awarded the Nobel Prize in Chemistry in 2000 for their discoveries.[5][6]. However, because of the numerous prior discoveries of highly-conductive polyacetylenes, their priority is disputed in textbooks and reviews[7] [8].
References
- ^ Jozefiak, T. H.; Ginsburg, E. J.; Gorman, C. B.; Grubbs, R. H.; Lewis, N. S."Voltammetric Characterization of Soluble Polyacetylene Derivatives Obtained from the Ring-Opening Metathesis Polymerization (ROMP) of Substituted Cyclooctatetraenes" Journal of the American Chemical Society 1993; volume 115, pages 4705-4713. doi:10.1021/ja00064a035
- ^ Gorman, C. B. Ginsburg, E. J.; Grubbs, R. H. "Soluble, Highly Conjugated Derivatives of Polyacetylene from the Ring-Opening Metathesis Polymerization of Monosubstituted Cyclooctatetraenes: Synthesis and the Relationship Between Polymer Structure and Physical Properties" Journal of the American Chemical Society 1993, volume 115, pages 1397-1409. doi:10.1021/ja00057a024
- ^ Langsdorf, Brandi, L.; Zhou, Xin; Lonergan, Mark C., "Kinetic Study of the Ring-Opening Metathesis Polymerization of Ionically Functionalized Cyclooctatetraenes" Macromolecules, 2001, volume 34, pages 2450-2458. doi:10.1021/ma0020685
- ^ Organic Semiconductors by Yoshikuko Okamoto and Walter Brenner, Reinhold (1964). Chapt.7, Polymers, pp125-158
- ^ Chiang, C. K.; Druy, M. A.; Gau, S. C.; Heeger, A. J.; Louis, E. J.; MacDiarmid, A. G.; Park, Y. W.; Shirakawa, H., "Synthesis of Highly Conducting Films of Derivatives of Polyacetylene, (CH)x," J. Am. Chem. Soc. 1978, 100, 1013-15. doi:10.1021/ja00471a081
- ^ Ebbing, Darrell (2005). General Chemistry (8th ed.). New York: Houghton Mifflin Company. pp. 1042–1043. ISBN 0-618-399410.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ An overview of the First Half-Century of Molecular Electronics" by Noel S. Hush, Ann. N.Y. Acad. Sci. 1006: 1–20 (2003).
- ^ Historical Background (or there is nothing new under the Sun), Inzelt,G. "Conducting Polymers", (2008), chapter 8, pp.265-269.
