5-Methylcytosine

| |
| Names | |
|---|---|
| IUPAC name
4-amino-5-methyl-3H-pyrimidin-2-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.236 |
| KEGG | |
| MeSH | 5-Methylcytosine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H7N3O | |
| Molar mass | 125.131 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
5-Methylcytosine is a methylated form of the DNA base cytosine that may be involved in the regulation of gene transcription. When cytosine is methylated, the DNA maintains the same sequence, but the expression of methylated genes can be altered (the study of this is part of the field of epigenetics). 5-Methylcytosine is incorporated in the nucleoside 5-methylcytidine.
In 5-methylcytosine, a methyl group, is attached to the 5th carbon atom (counting counterclockwise from the NH nitrogen at the six o'clock position, not the 2 o'clock). This methyl group distinguishes 5-methylcytosine from cytosine.
In vivo
5-Methylcytosine is an epigenetic modification formed by the action of DNA methyltransferases.

The function of this chemical varies significantly among species:[1]
- In bacteria, 5-methylcytosine can be found at a variety of sites, and is often used as a marker to protect DNA from being cut by native methylation-sensitive restriction enzymes.
- In plants, 5-methylcytosine occurs at CpG, CpHpG and CpHpH sequences (where H = A, C or T).
- In fungi and animals, 5-methylcytosine predominantly occurs at CpG dinucleotides. Most eukaryotes methylate only a small percentage of these sites, but 70-80% of CpG cytosines are methylated in vertebrates.
While spontaneous deamination of cytosine forms uracil, which is recognized and removed by DNA repair enzymes, deamination of 5-methylcytosine forms thymine. This conversion of a DNA base from cytosine (C) to thymine (T) can result in a transition mutation. In addition, active enzymatic deamination of cytosine or 5-methylcytosine by the APOBEC family of cytosine deaminases could have beneficial implications on various cellular processes as well as on organismal evolution.Cite error: The <ref> tag has too many names (see the help page). The implications of deamination on 5-hydroxymethylcytosine, on the other hand, remains less understood.
In a NIH-funded study (profiled by Marjorie Montemayor-Quellenberg on the Harvard Gazette's website, and published on Friday, September 14, 2012, in "Cell") conducted by teams of researchers in Boston, Massachusetts at Harvard University Medical School (HMS), and one of its affiliates, Brigham and Women's Hospital (BWH), led by HMS Assistant Professor of Medicine, Dr. Yujiang Geno Shi, Ph.D., and Dr. George F. Murray, M.D., of BWH's Department of Pathology, it was found that "loss of ... 5-hmC in skin cells serves as a key indicator for malignant melanoma. Loss corresponded to more-advanced stages of melanoma as well as clinical outcome... Strikingly, researchers were able to reverse melanoma growth in preclinical studies. ..." For more information, please see the link to the article.[2]
In vitro
The NH2 group can be removed (deamination) from 5-methylcytosine to form thymine with use of reagents such as nitrous acid; cytosine deaminates to uracil under similar conditions.
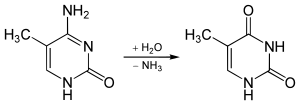
5-Methylcytosine is resistant to deamination by bisulfite treatment, which deaminates cytosine residues. This property is often exploited to analyze DNA cytosine methylation patterns with bisulfite sequencing.[3]
See also
References
- ^ Colot V, Rossignol JL (1999). "Eukaryotic DNA methylation as an evolutionary device". Bioessays. 21 (5): 402–411. doi:10.1002/(SICI)1521-1878(199905)21:5<402::AID-BIES7>3.0.CO;2-B. PMID 10376011.
- ^ http://news.harvard.edu/gazette/story/2012/09/skin-cancer-detection-breakthrough/
- ^ Clark SJ, Harrison J, Paul CL, Frommer M (1994). "High sensitivity mapping of methylated cytosines". Nucleic Acids Res. 22 (15): 2990–2997. doi:10.1093/nar/22.15.2990. PMC 310266. PMID 8065911.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Literature
- Griffiths, Anthony J. F. (1999). An Introduction to genetic analysis. San Francisco: W.H. Freeman. Chapter 15: Gene Mutation. ISBN 0-7167-3520-2.
{{cite book}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help) (available online at the United States National Center for Biotechnology Information)
