Carborane
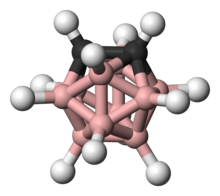
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms that may also contain other nonmetallic and nonmetallic elements in the cluster framework. [1] Like many of the related boron hydrides, these clusters are polyhedra or fragments of polyhedra, and are similarly classified as closo-, nido-, arachno-, hypho-, etc. based on whether they represent a complete (closo-) polyhedron, or a polyhedron that is missing one (nido-), two (arachno-), or more vertices. Carboranes are a notable example of heteroboranes.
Carboranes having as few as 5 and as many as 15 atoms in the cage framework are known, but the majority have two cage carbon atoms and obey the general formula C2BnHn+2 where n = 3 to 12. However, carboranes having 1 to 5 cage carbon atoms have been prepared, as well as carborane mono- and dianions. The icosahedral CB11H12(-) anion is a strong acid, and its polychlorinated derivative H(CHB11Cl11) is a superacid.
The icosahedral charge-neutral closo-carboranes, 1,2-, 1,7-, and 1,2-C2B10H12 (respectively ortho-, meta-, and para-carborane in the informal nomenclature) are particularly stable and are commercially available.[2] These boron-rich clusters exhibit unique organomimetic properties with chemical reactivity matching classical organic molecules, yet are structurally similar to metal-based inorganic and organometallic species[3]
The C2B10H12 isomers, together with other carborane clusters and metallacarboranes (see below) are utilized in a wide range of applications including heat-resistant polymers, recovery of radioactive metals from nuclear waste, catalysis, novel electroactive materials, and medical applications (see reference [1]). Like other electron-delocalized polyhedral clusters, the electronic structure of these cluster compounds can be described by Wade-Mingos rules.
As noted above, carboranes are closely related to boron hydrides (boranes), and thus are structurally very different from hydrocarbons, owing to the fact that carbon atoms have one more valence electron than do boron atoms (a notable feature of carboranes is that the cage carbon atoms often are directly bound to as many as 6, or even more, neighboring atoms, quite unlike the hydrocarbons). Carboranes and boranes adopt 3-dimensional cage (cluster) geometries in order to facilitate their electron-delocalized nonclassical bonding, whereas hydrocarbons are typically chains or rings; for example, B4H10 has an arachno cage geometry while the butane isomers n- and iso-C4H10, with 4 more electrons than B4H10, adopt linear or branched chain structures. Examples of nido and arachno- carbaboranes include 2,3-C2B4H8 and 1,3-C2B7H13, respectively. Geometrical isomers of carbaboranes may exist, necessitating the use of the numerical prefixes in a compound's name. For an overview of carborane chemistry see "Chemistry of the Elements", pp. 181–189[4] A comprehensive treatment of this area, updated annually, can be found in reference [1]. Electron-delocalized bonding in boranes is discussed extensively in [5]
Preparation
Carboranes are prepared by the addition of unsaturated carbon reagents to boron hydride clusters.
Monocarba derivatives
Monocarboranes are clusters with BnC cages. The 12-vertex derivative is best studied, but several are known.
Typically they are prepared by the addition of one-carbon reagents to boron hydride clusters. One-carbon reagents include cyanide, isocyanides, and formaldehyde. For example, monocarbadodecaborate ([CB11H12]-) is produced from decaborane and formaldehyde, followed by addition of borane dimethylsulfide.[6][7]
Dicarba derivatives
Dicarbaboranes can be prepared from boron hydride clusters with an alkyne as the source of the two carbon centers. Examples of some known closo-dicarbaboranes are C2B3H5, C2B6H8, C2B7H9, and C2B8H10.

The preparation of the most famous carboranes, closo-dicarbadodecaboranes, uses acetylene as the C2 source and decaborane and the source of B10:
The discovery of these compounds is complicated. In the late 1950's and early 1960's I was working at Reaction Motors doing classified contract work on decaborane derivitaives for the Air force. We sent bi-weekly reports to the Air Force which circulated them to other contractors doing similar research among which was Olin Matheson. We prepared the first carborane derivitive (isopropenyl carborane, which I purified for analysis. I then prepared diacetoxymethyl carborane and hydrolyzed it to dihydroxymethyl carborane. This was the first carborane derivitive containing an active functional group. We reported this in our regular reports, but saw no mention of work at Olin Matheson till later, when several meetings were held with the Air Force. After two to Three years this work was declassified and allowed to be published. A meeting was held with the editors of Inorganic Chemistry with the aim of arranging priorities. I do not know how it was decided that the Olin group was given priority (perhaps a coin toss) and I have lost touch with all the other authors, but I recently saw that our work was no longer credited and would like to correct this.[9]
Heying, T. L.; Ager, J. W.; Clark, S. L.; Mangold, D. J.; Goldstein, H. L.; Hillman, M.; Polak, R. J.; Szymanski, J. W. (1963). "A New Series of Organoboranes. I. Carboranes from the Reaction of Decaborane with Acetylenic Compounds". Inorganic Chemistry. 2 (6): 1089–1092. doi:10.1021/ic50010a002.</ref>
- nido-B10H14 + 2SEt2 → B10H12(SEt2)2 + H2
- B10H12(SEt2)2 + C2H2→ closo-1,2-C2B10H12 +2SEt2 + H2
It melts, without decomposition, at 320 °C.
Isomerism
Dicarbaboranes often exist as isomers that differ with respect to the relative location of the carbon centers. For example, the reaction of pentaborane(9) with acetylene affords all three isomers:
- nido-B5H9 + C2H2 closo-1,5-C2B3H5, closo-1,6-C2B4H6, closo-2,4-C2B5H7
When the reaction is conducted at a lower temperatures, a nido-intermediate is obtained:
- nido-B5H9 + C2H2 nido-2,4-C2B4H8
Other procedures carboranes that three carbon atoms or four carbon atoms.[10]
1,2-C2B10H12 rearranges to form the 1,3-isomer. The para- isomer is produced by heating to temperatures above 600 °C.
Reactions
Closo-dicarbadodecaboranes can undergo a wide variety of organic chemistry reactions. Deprotonation using organolithium reagents gives the dilithio derivatives.[11]
- C2B10H12 + 2 BuLi → Li2C2B10H10 + 2 BuH
These dilithiated compounds react with a variety of electrophiles, e.g. chlorophosphines, chlorosilanes, and sulfur.[12]
Base-induced degradation of carboranes give anionic nido derivatives, which are used as ligands for transition metals. The great majority of the work has been conducted on derivatives of ortho-carborane, but larger carboranes have also been investigated.[13]
Research
Dicarbollide complexes have been evaluated for many applications for many years, but commercial applications are rare. The bis(dicarbollide) [Co(C2B9H11)2]- has been used as a precipitant for removal of 137Cs+ from radiowastes.[14]
The medical applications of carboranes have been explored (see ref. [1], Chapter 16).[15] C-functionalized carboranes represent a source of boron for boron neutron capture therapy.[16]
Some metal complexes exhibit catalytic properties.[17]
The compound H(CHB11Cl11) is a superacid, forming a isolable salt with protonated benzene, C6H+
7.[18] It protonates fullerene, C60.[19]
See also
References
- ^ Grimes, R. N., Carboranes 3rd Ed., Elsevier, Amsterdam and New York (2016) ISBN=9780128018941
- ^ Jemmis, E. D. (1982). "Overlap control and stability of polyhedral molecules. Closo-Carboranes". Journal of the American Chemical Society. 104 (25): 7017–7020. doi:10.1021/ja00389a021.
- ^ Spokoyny, A. M. (2013). "New ligand platforms featuring boron-rich clusters as organomimetic substituents". Pure and Applied Chemistry. 85 (5): 903–919. doi:10.1351/PAC-CON-13-01-13. PMC 3845684.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Lipscomb W.N. Boron Hydrides. Benjamin, New York (1963)|
- ^ W. H. Knoth (1967). "1-B9H9CH- and B11H11CH-". J. Am. Chem. Soc. 89: 1274–1275. doi:10.1021/ja00981a048.
- ^ "Convenient Route to Monocarba-closo-dodecaborate Anions". Organometallics. 35: 2022–2025. 2016. doi:10.1021/acs.organomet.6b00309.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ G. S. Pawley (1966). "Further Refinements of Some Rigid Boron Compounds". Acta Crystallogr. 20: 631–638. doi:10.1107/S0365110X66001531.
- ^ J. Inorg Chem. II(6),1101,1115,1120,1125,(1963) Nelson. N. Schwartz.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. p183
- ^ "Influential Role of Ethereal Solvent on Organolithium Compounds: The Case of Carboranyllithium". Chemistry – A European Journal. 18: 3174–3184. 2012. doi:10.1002/chem.201102626.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Jin, G.-X. (2004). "Advances in the Chemistry of Organometallic Complexes with 1,2-Dichalcogenolato-o-Carborane Ligands". Coord. Chem. Rev. 248: 587–602. doi:10.1016/j.ccr.2004.01.002.
- ^ "Advances in the Chemistry of carboranes and metallacarboranes with more than 12 vertices". Coord. Chem. Rev. 251: 2452–2476. 2007. doi:10.1016/j.ccr.2007.02.009.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Cobalt bis(dicarbollide) anion and its derivatives". J. Organomet. Chem. 849–850: 170–194. 2017. doi:10.1016/j.jorganchem.2017.04.006.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Boron in drug discovery: carboranes as unique pharmacophores in biologically active compounds". Chem. Rev. 111: 5701–5722. 2011. doi:10.1021/cr2000866.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Soloway, A. H.; Tjarks, W.; Barnum, B. A.; Rong, F.-G.; Barth, R. F.; Codogni, I. M.; Wilson, J. G. (1998). "The Chemistry of Neutron Capture Therapy". Chemical Reviews. 98 (4): 1515–1562. doi:10.1021/cr941195u. PMID 11848941.
- ^ Crowther, D. J.; Baenziger, N. C.; Jordan, R. F. (1991). "Group 4 metal dicarbollide chemistry. Synthesis, structures and reactivity of electrophilic alkyl complexes (Cp*)(C2B9H11)M(R), M = Hf, Zr". Journal of the American Chemical Society. 113 (4): 1455–1457. doi:10.1021/ja00004a080.
- ^ Olah, G. A.; Prakash, G. K. S.; Sommer, J.; Molnar, A. (2009). Superacid Chemistry (2nd ed.). Wiley. p. 41. ISBN 978-0-471-59668-4.
- ^ Reed Christopher A (2013). "Myths about the Proton. The Nature of H+ in Condensed Media". Acc. Chem. Res. 46 (11): 2567–2575. doi:10.1021/ar400064q. PMC 3833890. PMID 23875729.


