Sugammadex
Sugammadex (designation Org 25969) is a novel agent for reversal of neuromuscular blockade by the agent rocuronium in general anaesthesia. Sugammadex is currently in Phase 3 study and has not been approved for use.
Sugammadex is a modified γ-cyclodextrin, with a lipophilic core and a hydrophilic periphery. This gamma cyclodextrin has been modified from its natural state by placing eight carboxyl thio ether groups at the sixth carbon positions. These extensions extend the cavity size allowing greater encapsulation of the rocuronium molecule. Additionally, these negatively charged extensions electrostatically bind to the positively charged ammonium group. This binding encapsulation has been found to be one of the strongest found among cyclodextrin and their guest molecules. The rocuronium molecule (a modified steroid) bound within sugammadex's lipophilic core, is rendered unavailable to bind to the acetyl choline receptor at the neuromuscular junction. Sugammadex binding to rocuronium has been found to be permanent and complete.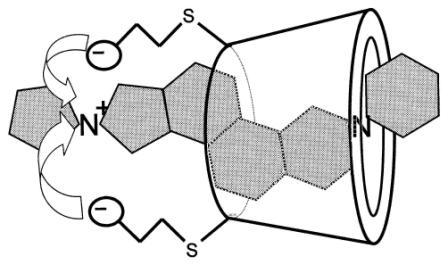
The main advantage of sugammadex is reversal of neuromuscular blockade without relying on inhibition of acetylcholinesterase. Therefore it does not cause the autonomic instability produced by anticholinesterases such as neostigmine, and antimuscarinic agents such as atropine do not need to be co-administered. Its administration is therefore associated with much greater cardiovascular and autonomic stability than the traditional reversal agents.
Recurarisation, a phenomenon of recurrence of neuromuscular block, may occur where the reversal agents wear off before a neuromuscular blocking drug is completely cleared. This is very unusual with all but the longest acting neuromuscular blocking drugs (such as gallamine, pancuronium or tubocurarine). It has not been demonstrated to occur with sugammadex."[1]
Sugammadex doses of 2-8mg/kg may be used for adults pending drug approval. Data for children are not yet available.
γ-cyclodextrins have eight glucose units around the central ring of the molecule. Other cyclodextrins have different numbers, and correspondingly different properties.
Sugammadex also has some affinity for the aminosteroid neuromuscular blocking agents vecuronium and pancuronium. Though sugammadex's affinity for vecuronium is lower than its affinity for rocuronium, reversal of vecuronium is still effective because less vecuronium molecules are present in vivo for equivalent blockade. Vecuronium is approximately seven times more potent than rocuronium and overall requires less molecules to induce blockade. Sugammadex encapsulates with a 1:1 ratio and therefore will adequately reverse vecuronium as there are less molecules to bind compared to rocuroinum. Sugammadex reversal of pancuronium is not effective and has not been extensively studied.[2]
References:
- ^ Shields M, Giovannelli M, Mirakhur RK, Moppett I, Adams J, Hermens Y (2006). "Org 25969 (sugammadex), a selective relaxant binding agent for antagonism of prolonged rocuronium-induced neuromuscular block". British Journal of Anaesthesia. 96 (1): 36–43.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
2.Welliver M. New drug Sugammadex; A selective relaxant binding agent. 2006. AANA J. 74(5): 357-363.
