Siloxane
A siloxane is a functional group in organosilicon chemistry with the Si–O–Si linkage. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H(OSiH2)nOH and (OSiH2)n.[1] Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen atom. The siloxane functional group forms the backbone of silicones, the premier example of which is polydimethylsiloxane.[2] The functional group R3SiO- (where the three Rs may be different) is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility. [3]

Structure
Siloxanes generally adopt structures expected for linked tetrahedral ("sp3-like") centers. The Si–O bond is 1.64 Å (vs Si–C distance of 1.92 Å) and the Si–O–Si angle is rather open at 142.5°.[4] By way of contrast, the C–O distance in a typical dialkyl ether is much shorter at 1.414(2) Å with a more acute C–O–C angle of 111°.[5] It can be appreciated that the siloxanes would have low barriers for rotation about the Si–O bonds as a consequence of low steric hindrance. This geometric consideration is the basis of the useful properties of some siloxane-containing materials, such as their low glass transition temperatures.
Synthesis of siloxanes

The main route to siloxane functional group is by hydrolysis of silicon chlorides:
- 2 R3Si–Cl + H2O → R3Si–O–SiR3 + 2 HCl
The reaction proceeds via the initial formation of silanols (R3Si–OH):
- R3Si–Cl + H2O → R3Si–OH + HCl
The siloxane bond can then form via a silanol + silanol pathway or a silanol + chlorosilane pathway:
- 2 R3Si–OH → R3Si–O–SiR3 + H2O
- R3Si–OH + 2 R3Si–Cl → R3Si–O–SiR3 + HCl
Hydrolysis of a silyldichloride can afford linear or cyclic products. Linear products are terminated with silanol groups:
- n R2Si(OH)2 → H(R2SiO)nOH + (n−1) H2O
Cyclic products have no silanol termini:
- n R2Si(OH)2 → (R2SiO)n + n H2O
The linear products, polydimethylsiloxane (PDMS), are of great commercial value. Their production requires the production of dimethylsilicon dichloride.
Starting from trisilanols, cages are possible, such as the species with the formula (RSi)nO3n/2 with cubic (n = 8) and hexagonal prismatic (n = 12). (RSi)8O12 structures. The cubic cages are cubane-type clusters, with silicon centers at the corners of a cube oxygen centres spanning each of the twelve edges.[7]
Reactions
Oxidation of organosilicon compounds, including siloxanes, gives silicon dioxide. This conversion is illustrated by the combustion of hexamethylcyclotrisiloxane:
- ((CH3)2SiO)3 + 12 O2 → 3 SiO2 + 6 CO2 + 9 H2O
Strong base degrades siloxane group, often affording siloxide salts:
- ((CH3)3Si)2O + 2 NaOH → 2 (CH3)3SiONa + H2O
This reaction proceeds by production of silanols. Similar reactions are used industrially to convert cyclic siloxanes to linear polymers.[2]
Uses
Polysiloxanes, upon combustion in an inert atmosphere, generally undergo pyrolysis to form silicon oxycarbide or silicon carbide (SiC). By exploiting this reaction, polysiloxanes have been used as preceramic polymers in various processes including additive manufacturing. The use of a poly-siloxane precursor in polymer derived ceramics allows the formation ceramic bodies with complex shapes, although the significant shrinkage in pyrolysis needs to be taken into account.
Cyclomethicones
Cyclomethicones are a group of methyl siloxanes, a class of liquid silicones (cyclic polydimethylsiloxane polymers) that possess the characteristics of low viscosity and high volatility as well as being skin emollients and in certain circumstances useful cleaning solvents.[8] Unlike dimethicones, which are linear siloxanes that do not evaporate, cyclomethicones are cyclic: both groups consist of a polymer featuring a monomer backbone of one silicon and two oxygen atoms bonded together, but instead of having a very long "linear" backbone surrounded by a series of methyl groups (which produces a clear, non-reactive, non-volatile liquid ranging from low to high viscosity), cyclomethicones have short backbones that make closed or nearly-closed rings or "cycles" with their methyl groups, giving them many of the same properties of dimethicones but making them much more volatile. They are used in many cosmetic products where eventual complete evaporation of the siloxane carrier fluid is desired. In this way they are useful for products like deodorants and antiperspirants which need to coat the skin but not remain tacky afterward.[9] Most cyclomethicone is manufactured by Dow Corning.[10] Cyclomethicones have been shown to involve the occurrence of silanols during biodegration in mammals.[11] The resulting silanols are capable of inhibiting hydrolytic enzymes such as thermolysin, acetylcholinesterase, however the doses required for inhibition are by orders of magnitude higher than the ones resulting from the accumulated exposure to consumer products containing cyclomethicones.[12]
Nomenclature
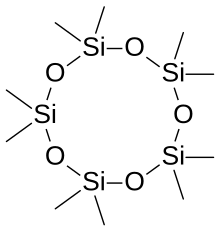
The word siloxane is derived from the words silicon, oxygen, and alkane. In some cases, siloxane materials are composed of several different types of siloxane groups; these are labeled according to the number of Si-O bonds. M-units: (CH3)3SiO0.5, D-units: (CH3)2SiO, T-units: (CH3)SiO1.5
| Cyclic siloxanes (cyclomethicones) | CAS | Linear siloxanes | CAS |
|---|---|---|---|
| L2, MM: hexamethyldisiloxane | 107-46-0 | ||
| D3: hexamethylcyclotrisiloxane | 541-05-9 | L3, MDM: octamethyltrisiloxane | 107-51-7 |
| D4: octamethylcyclotetrasiloxane | 556-67-2 | L4, MD2M: decamethyltetrasiloxane | 141-62-8 |
| D5: decamethylcyclopentasiloxane | 541-02-6 | L5, MD3M: dodecamethylpentasiloxane | 141-63-9 |
| D6: dodecamethylcyclohexasiloxane | 540-97-6 | L6, MD4M: tetradecamethylhexasiloxane | 107-52-8 |
Safety and environmental considerations
Because silicones are heavily used in biomedical and cosmetic applications, their toxicology has been intensively examined. "The inertness of silicones toward warmblooded animals has been demonstrated in a number of tests." With an LD50 in rats of >50 g/kg, they are virtually nontoxic.[13] Questions remain however about chronic toxicity or the consequences of bioaccumulation since siloxanes can be long-lived.
Findings about bioaccumulation have been largely based on laboratory studies. Field studies of bioaccumulation have not reached consensus. "Even if the concentrations of siloxanes we have found in fish are high compared to concentrations of classical contaminants like PCBs, several other studies in the Oslo Fjord in Norway, Lake Pepin in the US, and Lake Erie in Canada have shown concentrations of siloxanes decrease at higher range in the food chain. This finding raises questions about which factors influence the bioaccumulation potential of siloxanes."[14]
Cyclomethicones are ubiquitous because they are widely used in biomedical and cosmetic applications. They can be found at high levels in American cities. They can be toxic to aquatic animals in concentrations often found in the environment.[15][16] The cyclomethicones D4 and D5 are bioaccumulative in some aquatic organisms, according to one report.[17]
In the European Union, D4, D5 and D6 have been deemed hazardous as per the REACH regulation. They were characterized as substances of very high concern (SVHC) due to their PBT and vPvB properties.[18] Canada regulates D4 under a pollution prevention plan.[15] A scientific review in Canada in 2011 concluded that "Siloxane D5 does not pose a danger to the environment."[19]
Siloxanes also find their way to Renewable Natural Gas (RNG) sources like Landfill Gas (LFG) and Biogas from their use in numerous consumer products such as creams, detergents, deodorants and shampoos. If these gas sources are burnt in homes without treating them for siloxanes, it will produce silica particles which can be minute enough to enter the lungs and cause silicosis and other lung diseases like asthma [20][21][22]. These compounds when also used in industrial equipment such as heaters and boilers can cause them to clog and can damage them [20][21]. Therefore the RNG sources need to be treated for these siloxane compounds before being used in households. UV light combined with membrane reactors has been shown to degrade these siloxane compounds in RNG while preserving the methane (which is the useful energy component) inside it [23][24].
References
- ^ Siloxanes, IUPAC Gold Book
- ^ a b Röshe, L.; John, P.; Reitmeier, R. "Organic Silicon Compounds" Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley and Sons: San Francisco, 2003. doi:10.1002/14356007.a24_021.
- ^ "Siloxane". www.sciencedirect.com. Science Direct.
- ^ H. Steinfink, B. Post and I. Fankuchen "The crystal structure of octamethyl cyclotetrasiloxane" Acta Crystallogr. 1955, vol. 8, 420-424. doi:10.1107/S0365110X55001333
- ^ "Dichlorosilane–dimethyl ether aggregation: a new motif in halosilane adduct formation" K. Vojinović, U. Losehand, N. W. Mitzel Dalton Trans., 2004, 2578-2581. doi:10.1039/B405684A
- ^ Silicon: Organosilicon Chemistry. Encyclopedia of Inorganic Chemistry Online, 2nd ed.; Wiley: New Jersey, 2005. doi:10.1002/0470862106.ia220
- ^ S. D. Kinrade, J. C. H. Donovan, A. S. Schach and C. T. G. Knight (2002), Two substituted cubic octameric silicate cages in aqueous solution. J. Chem. Soc., Dalton Trans., 1250 - 1252. doi:10.1039/b107758a
- ^ Barbara Kanegsberg; Edward Kanegsberg (2011). Handbook for Critical Cleaning: Cleaning agents and systems. CRC. p. 19. ISBN 978-1-4398-2827-4.
- ^ Amarjit Sahota (25 November 2013). Sustainability: how the cosmetics industry is greening up. Wiley. p. 208. ISBN 978-1-118-67650-9.
- ^ Meyer Rosen (23 September 2005). Delivery System Handbook for Personal Care and Cosmetic Products: Technology, Applications and Formulations. William Andrew. p. 693. ISBN 978-0-8155-1682-8.
- ^ S. Varaprath, K. L. Salyers, K. P. Plotzke and S. Nanavati "Identification of Metabolites of Octamethylcyclotetrasiloxane (D4) in Rat Urine" Drug Metab Dispos 1999, 27, 1267-1273.
- ^ R. Pietschnig and S. Spirk: The Chemistry of Organo Silanetriols. Coord. Chem. Rev. 2016, 87-106. doi:10.1016/j.ccr.2016.03.010
- ^ Moretto, Hans-Heinrich; Schulze, Manfred; Wagner, Gebhard (2005). "Silicones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_057. ISBN 3527306730.
- ^ "Siloxanes: Soft, shiny -- and dangerous?" by Christine Solbakken, Science Nordic, August 28, 2015. Retrieved May 31, 2016
- ^ a b Karpus, Jennifer (20 June 2014). "Exec: Silicone industry must focus on safety, environment". Rubber & Plastic News. Retrieved 8 April 2015.
- ^ Bienkowski, Brian (30 April 2013). "Chemicals from Personal Care Products Pervasive in Chicago Air". Scientific American. Retrieved 8 April 2015.
- ^ Wang, De-Gao; Norwood, Warren; Alaee, Mehran; Byer, Jonatan D.; Brimble, Samantha (October 2013). "Review of recent advances in research on the toxicity, detection, occurrence and fate of cyclic volatile methyl siloxanes in the environment". Chemosphere. 93 (5): 711–725. Bibcode:2013Chmsp..93..711W. doi:10.1016/j.chemosphere.2012.10.041. PMID 23211328.
- ^ "Candidate List of substances of very high concern for Authorisation". ECHA. Retrieved 2019-12-18.
- ^ Report of the Board of Review for Decamethylcyclopentasiloxane (Siloxane D5) established under Section 333(1) of the Canadian Environmental Protection Act of 1999, October 20, 2011
- ^ a b Nair, Nitin; Zhang, Xianwei; Gutierrez, Jorge; Chen, Jack; Egolfopoulos, Fokion; Tsotsis, Theodore (2012-12-05). "Impact of Siloxane Impurities on the Performance of an Engine Operating on Renewable Natural Gas". Industrial & Engineering Chemistry Research. 51 (48): 15786–15795. doi:10.1021/ie302751n. ISSN 0888-5885.
- ^ a b Nair, Nitin; Vas, Arjun; Zhu, Tongyu; Sun, Wenjing; Gutierrez, Jorge; Chen, Jack; Egolfopoulos, Fokion; Tsotsis, Theodore T. (2013-05-08). "Effect of Siloxanes Contained in Natural Gas on the Operation of a Residential Furnace". Industrial & Engineering Chemistry Research. 52 (18): 6253–6261. doi:10.1021/ie400449y. ISSN 0888-5885.
- ^ Nair, Nitin. "Health and Environmental Risk of using Renewable Natural Gas in India" (PDF).
{{cite web}}: CS1 maint: url-status (link) - ^ [1], "Catalytic removal of gas phase contaminants", issued 2014-03-17
- ^ Nair, Nitin Narayanan (2013-07-04). Novel methods for landfill gas and biogas clean-up. University of Southern California. OCLC 857687061.
