Search results
Appearance
The page "Electron-transfer+reaction" does not exist. You can create a draft and submit it for review or request that a redirect be created, but consider checking the search results below to see whether the topic is already covered.
- of certain kinds of redox reactions involving transfer of electrons. Electrochemical processes are ET reactions. ET reactions are relevant to photosynthesis...6 KB (805 words) - 12:08, 3 May 2024
- called resonance energy transfer. If an electron of the special pair in the reaction center becomes excited, it cannot transfer this energy to another...28 KB (3,452 words) - 18:15, 8 August 2024
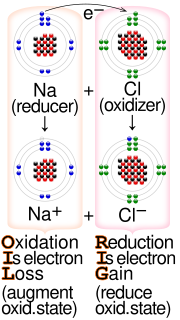
- Redox (redirect from One-electron reduction)chemical reaction. There are two classes of redox reactions: Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized...37 KB (3,575 words) - 19:47, 26 August 2024
- Marcus theory (section Inner sphere electron transfer)the rates of electron transfer reactions – the rate at which an electron can move or jump from one chemical species (called the electron donor) to another...40 KB (5,759 words) - 21:12, 17 April 2024
- acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions)...33 KB (4,063 words) - 11:38, 13 July 2024
- directly from sunlight or transferred as excitation energy via light-harvesting antenna systems, give rise to electron transfer reactions along the path of a...22 KB (2,798 words) - 18:17, 8 August 2024
- is generated, i.e., redox reaction takes place in excited state (this phenomenon is not observed in Dexter electron transfer). Such materials include semiconductors...4 KB (448 words) - 17:46, 21 September 2021
- Oxidizing agent (redirect from Electron acceptors)atom-transfer reactions. Electron acceptors participate in electron-transfer reactions. In this context, the oxidizing agent is called an electron acceptor...9 KB (875 words) - 20:49, 4 September 2024
- Inner sphere electron transfer (IS ET) or bonded electron transfer is a redox chemical reaction that proceeds via a covalent linkage—a strong electronic...4 KB (636 words) - 20:28, 22 July 2024
- A Proton-coupled electron transfer (PCET) is a chemical reaction that involves the transfer of electrons and protons from one atom to another. The term...8 KB (895 words) - 20:42, 15 January 2024
- exergonic reaction of forward electron transfer in the mitochondrial complex I when electrons travel from NADH to ubiquinone. The term "Reverse electron transfer"...7 KB (851 words) - 03:16, 19 May 2023
- potentials of 1-electron reduction of buckminsterfullerene and its anions is given in the table below: C60 forms a variety of charge-transfer complexes, for...46 KB (4,461 words) - 19:01, 19 August 2024
- Nobel Prize in Chemistry "for his contributions to the theory of electron transfer reactions in chemical systems". Marcus theory, named after him, provides...16 KB (1,383 words) - 16:32, 27 August 2024
- Outer sphere refers to an electron transfer (ET) event that occurs between chemical species that remain separate and intact before, during, and after...4 KB (541 words) - 07:57, 3 July 2023
- media. Electron-transfer theories describe the influence of a variety of parameters on the rate of electron-transfer. All electrochemical reactions occur...22 KB (2,221 words) - 02:16, 4 March 2024
- pericyclic reactions, there is often no obvious movement of electrons from an electron rich source to an electron poor sink. Rather, electrons are redistributed...10 KB (1,162 words) - 16:49, 21 April 2024
- Radical (chemistry) (redirect from Single electron transfer)or electron transfer, also known as reduction. Radicals are formed from other radicals through substitution, addition, and elimination reactions. Homolysis...40 KB (4,596 words) - 18:01, 3 September 2024
- formed and consumed. These reactions involve the transfer of electrons and the making/breaking of chemical bonds. Through reaction mechanisms, FAD is able...39 KB (4,310 words) - 22:40, 5 September 2024
- Sulfur (section Electron transfer reactions)redox reactions. Other examples include many zinc proteins, as well as iron–sulfur clusters. Most pervasive are the ferrodoxins, which serve as electron shuttles...99 KB (11,045 words) - 12:21, 4 September 2024
- In chemistry, charge-transfer (CT) complex, or electron donor-acceptor complex, describes a type of supramolecular assembly of two or more molecules or...12 KB (1,349 words) - 19:11, 5 June 2024
- electron transfer reaction (plural electron transfer reactions) (chemistry) a reaction in which a single electron is transferred from one molecule to another;
- begins in the conductor. Similarly the reaction at stopping the procession would drag the surrounding electrons with it. Accordingly the induced currents
- oxidation) reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons
- Inorganic Chemistry/Electron Transfer Reactions (4.7) Contents 1. Electron Transfer Reactions 2. Mechanism of Electron Transfer Reactions 2.1 Inner Sphere