3,5-Dinitrobenzoic acid
Appearance
You can help expand this article with text translated from the corresponding article in German. (April 2016) Click [show] for important translation instructions.
|

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
3,5-Dinitrobenzoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.501 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H4O6N2 | |
| Molar mass | 212.118 g/mol |
| Appearance | Yellow or colourless crystals |
| Melting point | 205 to 207 °C (401 to 405 °F; 478 to 480 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
3,5-Dinitrobenzoic acid is an organic chemical that is an important corrosion inhibitor and is also used in photography. This aromatic compound is used by chemists to identify alcohol components in esters and in the fluorometric analysis of creatinine.
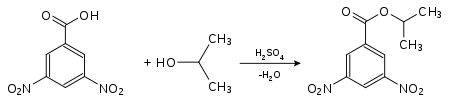
Identification of isopropanol as a derivate of 3,5-dinitrobenzoic acid:
3,5-dinitrobenzoic acid-2-propylester (mp.: 123 °C[1]).
References
- ^ CRC Handbook of Tables for Organic Compound Identification, Third Edition, 1984, ISBN 0-8493-0303-6.
Literature
- "3,5-dinitrobenzoic acid". Combined Chemical Dictionary. Chapman and Hall/CRC Press. 2007.
- B. C. Saunders, G. J. Stacey, I. G. E. Wilding: "The Preparation of 3:5-Dinitrobenzoic Acid and 3:5-Dinitrobenzoyl Chloride – Observations on the Acylation of Amino-acids by means of 3:5-Dinitrobenzoyl Chloride and certain other Acid Chlorides", Biochem. J., 1942, 36 (3–4), p. 368–375; Text; PDF.

