Trihalide
Appearance
This article needs additional citations for verification. (January 2021) |
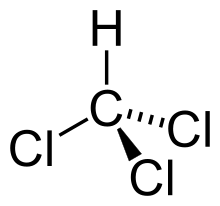
A trihalide in chemistry is an organohalide consisting of three halide atoms bonded to a single atom or compound.[1][2] An example of a trihalide is chloroform.
The trihalomethanes are the simplest trihalides, because only one hydrogen is connected to the carbon. The 1,1,1-Trichloroethane is one of the trihalides of ethane.
See also
[edit]References
[edit]- ^ "Definition of trihalide". Merriam Webster. merriam-webster.com. Retrieved 7 June 2017.
- ^ "Trihalides: Boron-Halogen Compounds READ FEEDBACK VERSION HISTORY USAGE". boundless.com. Retrieved 7 June 2017.
