Salinosporamide
Appearance
The salinosporamides are a group of closely related chemical compounds isolated from marine bacteria in the genus Salinispora.[1][2][3][4] They possess a densely functionalized γ-lactam-β-lactone bicyclic core.
Salinosporamide A has attracted interest for its potential use in treating various types of cancer.[5][6][7][8]
In addition, a variety of synthetic analogs have been prepared.[9]
Chemical structures
[edit]-
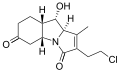
Salinosporamide B
-
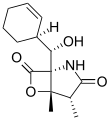
Salinosporamide C
-
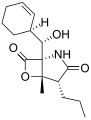
Salinosporamide D
-
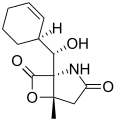
Salinosporamide E
-
Salinosporamide F
-
Salinosporamide G
-
Salinosporamide H
-
Salinosporamide I
-
Salinosporamide J
-
Salinosporamide K
References
[edit]- ^ Feling, Robert H.; Buchanan, Greg O.; Mincer, Tracy J.; Kauffman, Christopher A.; Jensen, Paul R.; Fenical, William (2003). "Salinosporamide A: A Highly Cytotoxic Proteasome Inhibitor from a Novel Microbial Source, a Marine Bacterium of the New Genus Salinospora". Angewandte Chemie International Edition. 42 (3): 355–7. doi:10.1002/anie.200390115. PMID 12548698.
- ^ Philip G. Williams; Greg O. Buchanan; Robert H. Feling; Christopher A. Kauffman; Paul R. Jensen & William Fenical (2005). "New Cytotoxic Salinosporamides from the Marine Actinomycete Salinispora tropica". J. Org. Chem. 70 (16): 6196–6203. doi:10.1021/jo050511+. PMID 16050677.
- ^ Reed, Katherine A.; Manam, Rama Rao; Mitchell, Scott S.; Xu, Jianlin; Teisan, Sy; Chao, Ta-Hsiang; Deyanat-Yazdi, Gordafaried; Neuteboom, Saskia T. C.; et al. (2007). "Salinosporamides D−J from the Marine ActinomyceteSalinispora tropica, Bromosalinosporamide, and Thioester Derivatives Are Potent Inhibitors of the 20S Proteasome". Journal of Natural Products. 70 (2): 269–76. doi:10.1021/np0603471. PMID 17243724.
- ^ Eustáquio, Alessandra S.; Nam, Sang-Jip; Penn, Kevin; Lechner, Anna; Wilson, Micheal C.; Fenical, William; Jensen, Paul R.; Moore, Bradley S. (2011). "The Discovery of Salinosporamide K from the Marine Bacterium "Salinispora pacifica" by Genome Mining Gives Insight into Pathway Evolution". ChemBioChem. 12 (1): 61–4. doi:10.1002/cbic.201000564. PMC 3088357. PMID 21154492.
- ^ Fenical, William; Jensen, Paul R.; Palladino, Michael A.; Lam, Kin S.; Lloyd, G. Kenneth; Potts, Barbara C. (2009). "Discovery and development of the anticancer agent salinosporamide A (NPI-0052)". Bioorganic & Medicinal Chemistry. 17 (6): 2175–80. doi:10.1016/j.bmc.2008.10.075. PMC 2814440. PMID 19022674.
- ^ Lam, Kin S.; Lloyd, G. Kenneth; Neuteboom, Saskia T. C.; Palladino, Michael A.; Sethna, Kobi M.; Spear, Matthew A.; Potts, Barbara C. (2009). "Chapter 12. From Natural Product to Clinical Trials: NPI-0052 (Salinosporamide A), a Marine Actinomycete-Derived Anticancer Agent". Natural Product Chemistry for Drug Discovery. p. 355. doi:10.1039/9781847559890-00355. ISBN 978-0-85404-193-0.
- ^ Gulder, Tobias A. M.; Moore, Bradley S. (2010). "Salinosporamide Natural Products: Potent 20 S Proteasome Inhibitors as Promising Cancer Chemotherapeutics". Angewandte Chemie International Edition. 49 (49): 9346–67. doi:10.1002/anie.201000728. PMC 3103133. PMID 20927786.
- ^ WO 2006118973, Palladino, Michael; Potts, Barbara Christine & Macherla, Venkata Rami Reddy et al., "Methods of using heterobyclic compounds for treatment of rectal cancer", published 2006-11-09, assigned to Nereus Pharmaceuticals Inc.
- ^ Nett, Markus; Gulder, Tobias A. M.; Kale, Andrew J.; Hughes, Chambers C.; Moore, Bradley S. (2009). "Function-Oriented Biosynthesis of β-Lactone Proteasome Inhibitors inSalinispora tropica". Journal of Medicinal Chemistry. 52 (19): 6163–7. doi:10.1021/jm901098m. PMC 2771571. PMID 19746976.
External links
[edit] Media related to Salinosporamides at Wikimedia Commons
Media related to Salinosporamides at Wikimedia Commons