Quebrachitol
| |
| Names | |
|---|---|
| IUPAC name
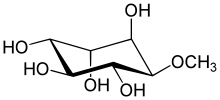
(1R,2S,4S,5R)-6-methoxycyclohexane-1,2,3,4,5-pentol
| |
| Other names
Quebrachitol
L-Quebrachitol (-)-Quebrachitol 2-O-methyl-l-inositol 2-0-methyl-chiro-inositol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H14O6 | |
| Molar mass | 194.18 g/mol |
| Appearance | White to off-white powder |
| Melting point | 190 to 198 °C (374 to 388 °F; 463 to 471 K) |
| Soluble in DMSO, dimethyl formamide, or water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Quebrachitol is a naturally occurring optically active cyclitol, a cyclic polyol. It can be found in Allophylus edulis[1] and in the serum left after the coagulation of the Hevea brasiliensis latex in the operation of rubber tapping.[2] It is also found in Cannabis sativa,[3] in Paullinia pinnata and in seabuckthorn.[4]
It was first isolated by Tanret in 1887 from the bark of Aspidosperma quebracho. The substance was tested as a sweetening agent for diabetics in 1933. It shows a sweetening property half of that of sucrose but induces colic or diarrhoea at concentration used to render the food palatable.[5]
Quebrachitol is a versatile building block in the construction of naturally occurring bioactive materials.[6] For example, its conversion into antifungal (E)-β-methoxyacrylate, oudemansin X has been made.[7]
References
- ^ First record of l-quebrachitol in Allophylus edulis (Sapindaceae). Martina Díaz, Andrés González, Ian Castro-Gamboa, David Gonzalez and Carmen Rossini, Carbohydrate Research, Volume 343, Issue 15, 13 October 2008, Pages 2699-2700, Read More}}
- ^ van Alphen, Jan (1951). "Quebrachitol". Industrial & Engineering Chemistry. 43: 141–145. doi:10.1021/ie50493a041.
- ^ 1955 - ACTA UNIVERSITATIS PALACKIANAE OLOMUCENSIS - TOM. VI. - HEMP AS A MEDICAMENT, Properties of isolated substances. Prof. Jan Kabelik, A brief survey of the methods of isolation and the physical and chemical properties and structures of the isolated antibacterial substances. F. Santavy & Z. Krejci
- ^ Some new data about antiviral and related activities of seabuckthorn principals and the prospects of their use. Shipulina L.D., All-Russian Research Institute of Medicinal and Aromatic Plants, Moscow, Russia
- ^ McCance, RA; Lawrence, RD (1933). "An investigation of quebrachitol as a sweetening agent for diabetics". Biochem J. 27 (4): 986–9. doi:10.1042/bj0270986. PMC 1252976. PMID 16745234.
- ^ Kiddle, James J. (1995). "Quebrachitol: A Versatile Building Block in the Construction of Naturally Occurring Bioactive Materials". Chemical Reviews. 95 (6): 2189–2202. doi:10.1021/cr00038a016.
- ^ Total synthesis of antibiotic (−)-oudemansin X utilizing L-quebrachitol as a chiral pool. Chida N., Yamada K. and Ogawa S., Chemistry Letters, 1992, no4, pp. 687-690
External links
- "Quebrachitol on the Sigma-Aldrich website". Archived from the original on October 3, 2012. Retrieved September 19, 2016.
{{cite web}}: CS1 maint: bot: original URL status unknown (link)
