Thioacetal
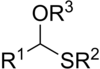

In organosulfur chemistry, thioacetals are the sulfur (thio-) analogues of acetals (R−CH(−OR)2). There are two classes: the less-common monothioacetals, with the formula R−CH(−OR')−SR", and the dithioacetals, with the formula R−CH(−SR')2 (symmetric dithioacetals) or R−CH(−SR')−SR" (asymmetric dithioacetals).[1]
The symmetric dithioacetals are relatively common. They are prepared by condensation of thiols (−SH) or dithiols (two −SH groups) with aldehydes (−CH=O). These reactions proceed via the intermediacy of hemithioacetals (R−CH(−OH)−SR'):
- Thiol addition to give hemithioacetal:
- Thiol addition with loss of water to give dithioacetal:
Such reactions typically employ either a Lewis acid or Brønsted acid as catalyst.
Dithioacetals generated from aldehydes and either 1,2-ethanedithiol or 1,3-propanedithiol are especially common among this class of molecules for use in organic synthesis.[2]
The carbonyl carbon of an aldehyde is electrophilic and therefore susceptible to attack by nucleophiles, whereas the analogous central carbon of a dithioacetal is not electrophilic. As a result, dithioacetals can serve as protective groups for aldehydes.
Far from being unreactive, and in a reaction unlike that of aldehydes, that carbon can be deprotonated to render it nucleophilic:
The inversion of polarity between R'(H)Cδ+=Oδ− and R'CLi(SR)2 is referred to as umpolung. The reaction is commonly performed using the 1,3-dithiane. The lithiated intermediate can be used for various nucleophilic bond-forming reactions, and then the dithioketal hydrolyzed back to its carbonyl form. This overall process, the Corey–Seebach reaction, gives the synthetic equivalent of an acyl anion.
See also
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "thioacetals". doi:10.1351/goldbook.T06348
- ^ P. Stütz And P. A. Stadler "3-alkylated And 3-acylated Indoles From A Common Precursor: 3-benzylindole And 3-benzoylindole" Org. Synth. 1977, 56, 8.doi:10.15227/orgsyn.056.0008




