3-Benzoxepin
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Benzoxepine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H8O | |
| Molar mass | 144.173 g·mol−1 |
| Appearance | Yellow solid[1] |
| Melting point | 84 (83–84 °C;[3] 84 °C[1]) |
| Solubility | soluble in apolar solvents (diethyl ether, benzene, tetrachloromethane)[2] and alcohols (methanol)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
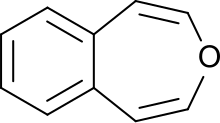
3-Benzoxepin is an annulated ring system with an aromatic benzene ring and a non-aromatic, unsaturated, oxygen-containing seven-membered heterocyclic oxepin. The first synthesis was described by Karl Dimroth and coworkers in 1961.[1] It is one of the three isomers of the benzoxepins.
Occurrence and synthesis
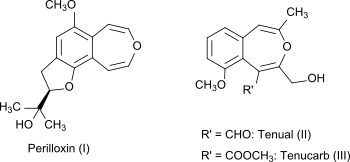
3-Benzoxepin itself is a non-natural compound, but the bicyclic ring system is part of the naturally occurring compounds perilloxin (I) from Perilla frutescens (variant acuta)[4] and tenual (II) and tenucarb (III) from Asphodeline tenuior.[5] Perilloxin inhibits the enzyme cyclooxygenase with an IC50 of 23.2 μM.[4] Non-steroidal anti-inflammatory drugs like aspirin and ibuprofen also work by inhibiting the cyclooxygenase enzyme family.[6]
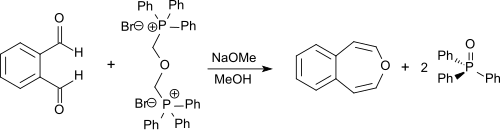
Unsubstituted 3-benzoxepin can be synthesized through a double Wittig reaction from o-phthalaldehyde with bis-(α,α′-triphenylphosphonium)-dimethylether-dibromide.[3] The latter compound can be synthesized from α,α′-dibromodimethyl ether (bis(bromomethyl)ether or BBME) which is accessible from hydrobromic acid, paraformaldehyde,[7] and triphenylphosphine. The reaction is performed in dry methanol with sodium methoxide, and the product is obtained in 55% yield.[1][2]
The compound can also be obtained through UV-irratiation of certain naphthalene derivatives such as 1,4-epoxy-1,4-dihydronaphthalene.[8]
It can also be obtained by photooxidation of 1,4-dihydronaphthalene, followed by pyrolysis of the formed hydroperoxides.[9]
The latter syntheses give 3-benzoxepins in low yields (4–6%).[8]
Properties
3-Benzoxepin is a bright yellow solid that crystallizes in platelets, with a smell similar to naphthalene. The material is soluble in apolar, organic solvents. Like naphthalene, it can be purified through sublimation. The solid is relatively acid-resistant, only under refluxing in concentrated, acidic alcohol solutions an unsaturated aldehyde is formed (likely an indene-3-aldehyde). Catalytic hydrogenation with a palladium catalyst results in 1,2,4,5-tetrahydro-3-benzoxepin.
References
- ^ a b c d Dimroth, K.; Pohl, G. (1961). "3-Benzoxepin". Angew. Chem. 73 (12): 436. Bibcode:1961AngCh..73..436D. doi:10.1002/ange.19610731215.
- ^ a b Rosowsky, A., ed. (1972). "II. Oxepin Ring Systems Containing Two Rings". Seven-Membered Heterocyclic Compounds Containing Oxygen and Sulfur. The Chemistry of Heterocyclic Compounds (in German). Vol. 26th. New York: Wiley-Interscience. p. 96. ISBN 0-471-38210-8.
- ^ a b c Dimroth, K.; Pohl, G.; Follmann, H. (1966). "Die Synthese von Derivaten des 3-Oxepins und des Furans durch eine zweifache Wittig-Reaktion". Chem. Ber. (in German). 99 (2): 634–641. doi:10.1002/cber.19660990238.
- ^ a b Liu, J.-H.; Steigel, A.; Reininger, E.; Bauer, R. (2000). "Two new prenylated 3-benzoxepin derivatives as cyclooxygenase inhibitors from Perilla frutescens var. acuta". J. Nat. Prod. 63 (3): 403–405. doi:10.1021/np990362o. PMID 10757731.
- ^ Ulubelen, A.; Tuzlaci, E.; Atilan, N. (1989). "Oxepine derivatives and anthraquinones from Asphodeline tenuior and A. taurica". Phytochemistry. 28 (2): 649–650. doi:10.1016/0031-9422(89)80076-7.
- ^ Kester, M.; Karpa, K. D.; Vrana, K. E. (2011). "NSAIDs". Pharmacology. Elsevier's Integrated Review. Elsevier Health Sciences. pp. 165–166. ISBN 9780323074452.
- ^ US patent 20040242799, Grabarnick, M. & Sasson, Y., "Process to bromomethylate aromatic compounds", published 2004-12-02, assigned to Grabarnick, M. and Sasson, Y.
- ^ a b Ziegler, G. R. (1969). "Mechanisms of photochemical reactions in solution. LVII. Photorearrangement of 1,4-epoxy-1,4-dihydronaphthalene to benz[f]oxepin". J. Am. Chem. Soc. 91 (2): 446–449. doi:10.1021/ja01030a040.
- ^ Jeffrey, A. M.; Jerina, D. M. (1972). "Autoxidation of 1,4-dihydronaphthalene. Formation of 3-benzoxepin via pyrolysis of 2-hydroperoxy-1,2-dihydronaphthalene". J. Am. Chem. Soc. 94 (11): 4048–4049. doi:10.1021/ja00766a084.