HMG-box
| HMG (high mobility group) box | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
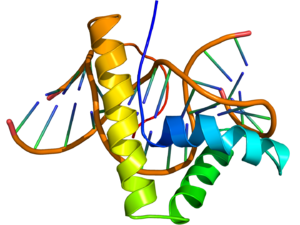 NMR structure of the HMG-box domain of the LEF1 protein (rainbow colored, N-terminus = blue, C-terminus = red) complexed with DNA (brown) based on the PDB: 2LEF coordinates. | |||||||||||
| Identifiers | |||||||||||
| Symbol | PF00505 | ||||||||||
| Pfam | PF00505 | ||||||||||
| InterPro | IPR009071 | ||||||||||
| SCOP2 | 1hsm / SCOPe / SUPFAM | ||||||||||
| |||||||||||
In molecular biology, the HMG-box (high mobility group box) is a protein domain which is involved in DNA binding.[1] The domain is composed of approximately 75 amino acid residues that collectively mediate the DNA-binding of chromatin-associated high-mobility group proteins. HMG-boxes are present in many transcription factors and chromatin-remodeling complexes, where they can mediate non-sequence or sequence-specific DNA binding.[2]
Structure
The structure of the HMG-box domain contains three alpha helices separated by loops (see figure to the right).[3]
Function
HMG-box containing proteins only bind non-B-type DNA conformations (kinked or unwound) with high affinity.[1] HMG-box domains are found in some high mobility group proteins, which are involved in the regulation of DNA-dependent processes such as transcription, replication, and DNA repair, all of which require changing the conformation of chromatin.[3] The single and the double box HMG proteins alter DNA architecture by inducing bends upon binding.[4][5]
References
- ^ a b Stros M, Launholt D, Grasser KD (October 2007). "The HMG-box: a versatile protein domain occurring in a wide variety of DNA-binding proteins". Cell. Mol. Life Sci. 64 (19–20): 2590–606. doi:10.1007/s00018-007-7162-3. PMID 17599239. S2CID 28156847.
- ^ Štros, M.; Launholt, D.; Grasser, K. D. (October 2007). "The HMG-box: a versatile protein domain occurring in a wide variety of DNA-binding proteins". Cellular and Molecular Life Sciences. 64 (19–20): 2590–2606. doi:10.1007/s00018-007-7162-3. Retrieved 18 February 2023.
- ^ a b Thomas JO (August 2001). "HMG1 and 2: architectural DNA-binding proteins". Biochem. Soc. Trans. 29 (Pt 4): 395–401. doi:10.1042/BST0290395. PMID 11497996.
- ^ D. Murugesapillai et al, DNA bridging and looping by HMO1 provides a mechanism for stabilizing nucleosome-free chromatin, Nucleic Acids Res (2014) 42 (14): 8996-9004
- ^ D. Murugesapillai et al, Single-molecule studies of high-mobility group B architectural DNA bending proteins, Biophys Rev (2016) doi:10.1007/s12551-016-0236-4
External links
- HMG-Box Domains at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
