MIL-53

MIL-53 (MIL ⇒ Matériaux de l′Institut Lavoisier) belongs to the class of metal-organic framework (MOF) materials. The first synthesis and the name was established by the group of Gérard Férey in 2002.[1] The MIL-53 structure consists of inorganic [M-OH] chains, which are connected to four neighboring inorganic chains by therephthalate-based linker molecules. Each metal center is octahedrally coordinated by six oxygen atoms. Four of these oxygen atoms originate from four different carboxylate groups and the remaining two oxygen atoms belong to two different μ-OH moieties, which bridge neighboring metal centers. The resulting framework structure contains one-dimensional diamond-shaped pores. Many research group have investigated the flexibility of the MIL-53 structure. This flexible behavior, during which the pore cross-section changes reversibly, was termed 'breathing effect' and describes the ability of the MIL-53 framework to respond to external stimuli.[2]
Structural Analogs
[edit]Monometallic single-linker MIL-53 analogs
[edit]MIL-53(Cr) was the first reported member of the MIL-53 family and is built up from Cr3+ as metal center and terephthalate (benzene-1,4-dicarboxylate) as linker molecules.[1] Based on the toolbox-like design of metal-organic framework materials, different metal centers or linker molecules can be used for the synthesis of other members of the MIL-53 family.[2] Trivalent (M3+) metal centers are mainly used, but materials with divalent (M2+) or tetravalent (M4+) metals have also been published.
| Name | Metal center and
oxidation state |
Year of
publication |
Alternative
name |
Reference |
|---|---|---|---|---|
| MIL-53(V) | V3+ | 2002 | MIL-47 | [3][4] |
| V4+ | ||||
| MIL-53(Cr) | Cr3+ | 2002 | [5][1] | |
| MIL-53(Al) | Al3+ | 2004 | [6] | |
| MIL-53(Fe) | Fe3+ | 2005 | [7] | |
| Fe2+ | 2005 | [7] | ||
| MIL-53(In) | In3+ | 2005 | [8] | |
| MIL-53(Co) | Co2+ | 2005 | MOF-71 | [9][10] |
| MIL-53(Ga) | Ga3+ | 2008 | [11] | |
| MIL-53(Mn) | Mn2+ | 2010 | [12] | |
| MIL-53(Sc) | Sc3+ | 2011 | [13] | |
| MIL-53(Ni) | Ni2+ | 2013 | [10] |
Terephthalate was used as linker molecules in the early reports on MIL-53 materials.[1] Later, terephthalate-based linker molecules with additional functional groups were used for the synthesis of functionalized MIL-53 materials.[2] Apart from the two carboxylate groups of terephthalate, which are used for the formation of the framework structure, the functional linker molecules contain one or more functional groups at the benzene ring, which do not participate in the formation of the framework.
| Functional linker | Metal center(M) | |||||
|---|---|---|---|---|---|---|
| V | Cr | Al | Fe | In | Ga | |

2-Aminobenzene-1,4-dicarboxylate |
[14] | - | [15][16] | [17] | [18] | [18] |

2-Fluorobenzene-1,4-dicarboxylate |
[19] | - | [19] | - | - | - |

2-Chlorobenzene-1,4-dicarboxylate |
[20] | [21] | [22] | [23] | - | - |

2-Bromobenzene-1,4-dicarboxylate |
[20] | - | [22] | [23] | [24] | - |
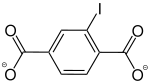
2-Iodobenzene-1,4-dicarboxylate |
- | - | [25] | - | - | - |

2-Nitrobenzene-1,4-dicarboxylate |
- | - | [22] | - | [24] | - |

Benzene-1,2,4-tricarboxylate |
- | - | [26] | - | - | - |

2-Methylbenzene-1,4-dicarboxylate |
[20] | [21] | [22] | [23] | - | - |

2-Trifluormethylbenzene-1,4-dicarboxylate |
[20] | - | - | - | - | - |

2-Hydroxybenzene-1,4-dicarboxylate |
[20] | - | [27] | - | - | - |

2-Methoxybenzene-1,4-dicarboxylate |
[20] | - | - | - | - | - |

2-Sulfobenzene-1,4-dicarboxylate |
- | - | [28] | - | - | - |

2-Isocyanatbenzene-1,4-dicarboxylate |
- | - | [29] | - | - | - |

2-Isothiocyanatbenzene-1,4-dicarboxylate |
- | - | [29] | - | - | - |

2,5-Dimethylbenzene-1,4-dicarboxylate |
[30] | - | - | - | - | - |
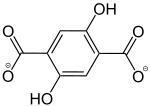
2,5-Dihydroxybenzene-1,4-dicarboxylate |
[30] | - | [22] | [23] | [24] | - |

2,5-Dithiolbenzene-1,4-dicarboxylate |
- | - | [31] | - | - | - |

2,5-Difluorobenzene-1,4-dicarboxylate |
[32] | - | [32] | - | - | - |
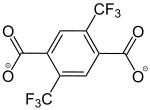
2,5-Bis(trifluormethyl)benzene-1,4-dicarboxylate |
[33] | - | - | [23] | - | - |

2-Amino-5-nitrobenzene-1,4-dicarboxylate |
- | - | [34] | - | [34] | [34] |

Benzene-1,2,4,5-tetracarboxylate |
- | - | [35]
MIL-121 |
[36] MIL-82 | - | - |

2,3,5,6-tetramethylbenzene-1,4-dicarboxylate |
- | [37]
MIL-105 |
- | - | - | - |

2,3,5,6-Tetrachlorobenzene-1,4-dicarboxylate |
[30] | - | - | - | - | - |

2,3,5,6-Tetrabromobenzene-1,4-dicarboxylate |
[30] | - | - | - | - | - |

Naphthalene-1,4-dicarboxylate |
[30] | - | [38] | - | - | - |
Mixed-component MIL-53 analogs
[edit]Apart from monometallic single-linker MIL-53 analogs, which contain one type of metal and one type of linker within the framework structure, several mixed-component MIL-53 analogs were reported. In mixed-metal MIL-53 materials, two different metals are incorporated into the framework structure at crystallographically equivalent lattice positions. Since both type of metals occupy equivalent positions, the metal ratio can usually be changed independent from the framework structure. Mixed-metal MIL-53 analogs have been synthesized mainly by direct synthesis procedures under hydrothermal or solvothermal conditions.
| Metal centers and
oxidation states |
Metal ratios
[-] |
Synthesis method | Citation |
|---|---|---|---|
| Al3+ / Cr3+ | 0.99 : 0.01 | Direct synthesis
hydrothermal |
[39][40][41] |
| Al3+ / V4+ | 0.99 : 0.01
0.95 : 0.05 0.71 : 0.29 0.32 : 0.68 0.13 : 0.87 |
Direct synthesis
hydrothermal |
[42] |
| ? | ? | [43] | |
| Al3+ / Fe3+ | 0.85 : 0.15
0.99 : 0.01 |
Direct synthesis | [44] |
| 0.96 : 0.04 | Post-synthetic metal-exchange | ||
| Al3+ / Ga3+ | ≈ 0.70 : 0.30
≈ 0.85 : 0.15 |
Direct synthesis
hydrothermal |
[45] |
| Cr3+ / V/4+ | 0.05 : 0.95
0.10 : 0.90 0.23 : 0.77 0.50 : 0.50 0.75 : 0.25 |
Direct synthesis
microwave |
[46] |
| 0.07 : 0.93
0.13 : 0.87 0.17 : 0.83 0.37 : 0.63 0.58 : 0.42 |
Direct synthesis
solvothermal | ||
| Cr3+ / Fe3+ | 0.60 : 0.40 | Direct synthesis
hydrothermal |
[47] |
| Fe2+/3+ / V2+/3+ | 0.88 : 0.12
0.76 : 0.24 0.74 : 0.26 0.49 : 0.51 |
Direct synthesis
solvothermal |
[48] |
| Fe2+ / Mn2+ | 0.90 : 0.10
0.88 : 0.12 0.82 : 0.18 0.74 : 0.26 |
Direct synthesis
solvothermal |
[49] |
| Fe2+ / Co2+ | 0.97 : 0.03
0.94 : 0.06 0.90 : 0.10 | ||
| Fe2+ / Ni2+ | 0.91 : 0.09
0.89 : 0.11 0.84 : 0.16 0.78 : 0.22 |
Similar to mixed-metal MIL-53 materials, mixed-linker MIL-53 analogs have been reported, in which two different linker molecules are incorporated into the framework structure at crystallographically equivalent positions with different ratios. One benefit of using the mixed-linker concept is that the number of functional groups in the framework can be adjusted by using different linker ratios.
| Linker 1 | Linker 2 | Linker ratios
[-] |
Metal center | Synthesis method | Citation |
|---|---|---|---|---|---|

Benzene-1,4-dicarboxylate |

2-Aminobenzene-1,4-dicarboxylate |
0.90 : 0.10
0.50 : 0.50 0.10 : 0.90 |
Al3+ | Direct synthesis
hydrothermal |
[50] |
| 0.90 : 0.10
0.82 : 0.18 0.51 : 0.49 0.48 : 0.62 |
[51][52][53] | ||||

Benzene-1,4-dicarboxylate |

2,5-Dihydroxybenzene-1,4-dicarboxylate |
0.75 : 0.25
0.50 : 0.50 0.25 : 0.75 |
Al3+ | Direct synthesis
solvothermal |
[54][55] |

Benzene-1,4-dicarboxylate |

2-Iodobenzene-1,4-dicarboxylate |
0.81 : 0.19
0.55 : 0.45 0.27 : 0.73 |
Al3+ | Direct synthesis
hydrothermal |
[25] |
References
[edit]- ^ a b c d Franck Millange, Christian Serre, Gérard Férey (2002-04-11), "Synthesis, structure determination and properties of MIL-53as and MIL-53ht: the first Criii hybrid inorganic–organic microporous solids: Criii(OH)·{O2C–C6H4–CO2}·{HO2C–C6H4–CO2H}xElectronic supplementary information (ESI) available: crystal data, atomic coordinates and metrical parameters for MIL-53as and MIL-53ht.", Chemical Communications (in German), no. 8, pp. 822–823, doi:10.1039/b201381a, PMID 12132491
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c Millange, Franck; Walton, Richard I. (2018-09-03). "MIL-53 and its Isoreticular Analogues: a Review of the Chemistry and Structure of a Prototypical Flexible Metal-Organic Framework". Israel Journal of Chemistry. 58 (9–10): 1019–1035. doi:10.1002/ijch.201800084. S2CID 105480508.
- ^ Karin Barthelet, Jérôme Marrot, Didier Riou, Gérard Férey (2002), "A Breathing Hybrid Organic–Inorganic Solid with Very Large Pores and High Magnetic Characteristics", Angewandte Chemie International Edition (in German), vol. 41, no. 2, pp. 281–284, doi:10.1002/1521-3773(20020118)41:2<281::AID-ANIE281>3.0.CO;2-Y, ISSN 1521-3773, PMID 12491409
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Hervé Leclerc, Thomas Devic, Sabine Devautour-Vinot, Philippe Bazin, Nathalie Audebrand (2011-10-13), "Influence of the Oxidation State of the Metal Center on the Flexibility and Adsorption Properties of a Porous Metal Organic Framework: MIL-47(V)", The Journal of Physical Chemistry C (in German), vol. 115, no. 40, pp. 19828–19840, doi:10.1021/jp206655y, ISSN 1932-7447
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ C. Serre, F. Millange, C. Thouvenot, M. Noguès, G. Marsolier, D. Louër, and G. Férey: Very Large Breathing Effect in the First Nanoporous Chromium(III)-Based Solids: MIL-53 or CrIII(OH)·{O2C-C6H4-CO2}·{HO2C-C6H4-CO2H}x·H2Oy. In: J. Am. Chem. Soc. 2002, 124, 45, S. 13519–13526, doi:10.1021/ja0276974.
- ^ Loiseau, Thierry; Serre, Christian; Huguenard, Clarisse; Fink, Gerhard; Taulelle, Francis; Henry, Marc; Bataille, Thierry; Férey, Gérard (2004-03-19). "A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration". Chemistry - A European Journal. 10 (6): 1373–1382. doi:10.1002/chem.200305413. ISSN 0947-6539. PMID 15034882.
- ^ a b Tabatha R. Whitfield, Xiqu Wang, Lumei Liu, Allan J. Jacobson (September 2005), "Metal-organic frameworks based on iron oxide octahedral chains connected by benzenedicarboxylate dianions", Solid State Sciences (in German), vol. 7, no. 9, pp. 1096–1103, Bibcode:2005SSSci...7.1096W, doi:10.1016/j.solidstatesciences.2005.03.007
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Ekaterina V. Anokhina, Marie Vougo-Zanda, Xiqu Wang, Allan J. Jacobson (2005-10-07), "In(OH)BDC·0.75BDCH 2 (BDC = Benzenedicarboxylate), a Hybrid Inorganic−Organic Vernier Structure", Journal of the American Chemical Society (in German), vol. 127, no. 43, pp. 15000–15001, doi:10.1021/ja055757a, ISSN 0002-7863, PMID 16248619
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Nathaniel L. Rosi, Jaheon Kim, Mohamed Eddaoudi, Banglin Chen, Michael O'Keeffe (2005-01-13), "Rod Packings and Metal−Organic Frameworks Constructed from Rod-Shaped Secondary Building Units", Journal of the American Chemical Society (in German), vol. 127, no. 5, pp. 1504–1518, doi:10.1021/ja045123o, ISSN 0002-7863, PMID 15686384
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Alexis S. Munn, Guy J. Clarkson, Franck Millange, Yves Dumont, Richard I. Walton (2013), "M(ii) (M = Mn, Co, Ni) variants of the MIL-53-type structure with pyridine-N-oxide as a co-ligand", CrystEngComm (in German), vol. 15, no. 45, p. 9679, doi:10.1039/c3ce41268g, ISSN 1466-8033
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Marie Vougo-Zanda, Jin Huang, Ekaterina Anokhina, Xiqu Wang, Allan J. Jacobson (2008-12-15), "Tossing and Turning: Guests in the Flexible Frameworks of Metal(III) Dicarboxylates", Inorganic Chemistry (in German), vol. 47, no. 24, pp. 11535–11542, doi:10.1021/ic800008f, ISSN 0020-1669, PMID 18433098
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Guohai Xu, Xiaoguang Zhang, Peng Guo, Chengling Pan, Hongjie Zhang (2010-03-24), "Mn II -based MIL-53 Analogues: Synthesis Using Neutral Bridging μ 2 -Ligands and Application in Liquid-Phase Adsorption and Separation of C6−C8 Aromatics", Journal of the American Chemical Society (in German), vol. 132, no. 11, pp. 3656–3657, doi:10.1021/ja910818a, ISSN 0002-7863, PMID 20196605
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Mowat, John P.S.; Miller, Stuart R.; Slawin, Alexandra M.Z.; Seymour, Valerie R.; Ashbrook, Sharon E.; Wright, Paul A. (June 2011). "Synthesis, characterisation and adsorption properties of microporous scandium carboxylates with rigid and flexible frameworks". Microporous and Mesoporous Materials. 142 (1): 322–333. doi:10.1016/j.micromeso.2010.12.016.
- ^ Karen Leus, Sarah Couck, Matthias Vandichel, Gauthier Vanhaelewyn, Ying-Ya Liu (2012), "Synthesis, characterization and sorption properties of NH2-MIL-47", Physical Chemistry Chemical Physics (in German), vol. 14, no. 44, p. 15562, Bibcode:2012PCCP...1415562L, doi:10.1039/c2cp42137b, ISSN 1463-9076, PMID 23073025
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Tim Ahnfeldt, Daniel Gunzelmann, Thierry Loiseau, Dunja Hirsemann, Jürgen Senker (2009-04-06), "Synthesis and Modification of a Functionalized 3D Open-Framework Structure with MIL-53 Topology", Inorganic Chemistry (in German), vol. 48, no. 7, pp. 3057–3064, doi:10.1021/ic8023265, hdl:10536/DRO/DU:30064444, ISSN 0020-1669, PMID 19245258
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Tim Ahnfeldt, Nathalie Guillou, Daniel Gunzelmann, Irene Margiolaki, Thierry Loiseau (2009-06-29), "[Al 4 (OH) 2 (OCH 3 ) 4 (H 2 N-bdc) 3 ]⋅ x H 2 O: A 12-Connected Porous Metal-Organic Framework with an Unprecedented Aluminum-Containing Brick", Angewandte Chemie International Edition (in German), vol. 48, no. 28, pp. 5163–5166, doi:10.1002/anie.200901409, PMID 19504512
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Sebastian Bauer, Christian Serre, Thomas Devic, Patricia Horcajada, Jérôme Marrot (2008-08-06), "High-Throughput Assisted Rationalization of the Formation of Metal Organic Frameworks in the Iron(III) Aminoterephthalate Solvothermal System", Inorganic Chemistry (in German), vol. 47, no. 17, pp. 7568–7576, doi:10.1021/ic800538r, ISSN 0020-1669, PMID 18681423
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Pablo Serra-Crespo, Elena Gobechiya, Enrique V. Ramos-Fernandez, Jana Juan-Alcañiz, Alberto Martinez-Joaristi (2012-09-04), "Interplay of Metal Node and Amine Functionality in NH 2 -MIL-53: Modulating Breathing Behavior through Intra-framework Interactions", Langmuir (in German), vol. 28, no. 35, pp. 12916–12922, doi:10.1021/la302824j, ISSN 0743-7463, PMID 22891682
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Shyam Biswas, Tom Rémy, Sarah Couck, Dmytro Denysenko, Geert Rampelberg (2013), "Partially fluorinated MIL-47 and Al-MIL-53 frameworks: influence of functionalization on sorption and breathing properties", Physical Chemistry Chemical Physics (in German), vol. 15, no. 10, p. 3552, Bibcode:2013PCCP...15.3552B, doi:10.1039/c3cp44204g, ISSN 1463-9076, PMID 23381460
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Shyam Biswas, Danny E. P. Vanpoucke, Toon Verstraelen, Matthias Vandichel, Sarah Couck (2013-11-07), "New Functionalized Metal–Organic Frameworks MIL-47-X (X = −Cl, −Br, −CH 3, −CF 3, −OH, −OCH 3 ): Synthesis, Characterization, and CO 2 Adsorption Properties", The Journal of Physical Chemistry C (in German), vol. 117, no. 44, pp. 22784–22796, doi:10.1021/jp406835n, ISSN 1932-7447
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Pascal G. Yot, Ke Yang, Vincent Guillerm, Florence Ragon, Vladimir Dmitriev (September 2016), "Impact of the Metal Centre and Functionalization on the Mechanical Behaviour of MIL-53 Metal-Organic Frameworks: Impact of the Metal Centre and Functionalization on the Mechanical Behaviour of MIL-53 Metal-Organic Frameworks", European Journal of Inorganic Chemistry (in German), vol. 2016, no. 27, pp. 4424–4429, doi:10.1002/ejic.201600263, hdl:10023/11147, S2CID 100565312
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Shyam Biswas, Tim Ahnfeldt, Norbert Stock (2011-10-03), "New Functionalized Flexible Al-MIL-53-X (X = -Cl, -Br, -CH 3, -NO 2, -(OH) 2 ) Solids: Syntheses, Characterization, Sorption, and Breathing Behavior", Inorganic Chemistry (in German), vol. 50, no. 19, pp. 9518–9526, doi:10.1021/ic201219g, ISSN 0020-1669, PMID 21899293
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Thomas Devic, Patricia Horcajada, Christian Serre, Fabrice Salles, Guillaume Maurin (2010-01-27), "Functionalization in Flexible Porous Solids: Effects on the Pore Opening and the Host−Guest Interactions", Journal of the American Chemical Society (in German), vol. 132, no. 3, pp. 1127–1136, doi:10.1021/ja9092715, ISSN 0002-7863, PMID 20038143
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c Lei Wu, Gérald Chaplais, Ming Xue, Shilun Qiu, Joël Patarin (2019), "New functionalized MIL-53(In) solids: syntheses, characterization, sorption, and structural flexibility", RSC Advances (in German), vol. 9, no. 4, pp. 1918–1928, Bibcode:2019RSCAd...9.1918W, doi:10.1039/C8RA08522F, ISSN 2046-2069, PMC 9059721, PMID 35516115
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Tahmouresilerd, Babak; Larson, Patrick J.; Unruh, Daniel K.; Cozzolino, Anthony F. (2018-08-28). "Make room for iodine: systematic pore tuning of multivariate metal–organic frameworks for the catalytic oxidation of hydroquinones using hypervalent iodine". Catalysis Science & Technology. 8 (17): 4349–4357. doi:10.1039/C8CY00794B. ISSN 2044-4761.
- ^ Nele Reimer, Barbara Gil, Bartosz Marszalek, Norbert Stock (2012), "Thermal post-synthetic modification of Al-MIL-53–COOH: systematic investigation of the decarboxylation and condensation reaction", CrystEngComm (in German), vol. 14, no. 12, p. 4119, doi:10.1039/c2ce06649a, ISSN 1466-8033
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Dieter Himsl, Dirk Wallacher, Martin Hartmann (2009-06-08), "Improving the Hydrogen-Adsorption Properties of a Hydroxy-Modified MIL-53(Al) Structural Analogue by Lithium Doping", Angewandte Chemie International Edition (in German), vol. 48, no. 25, pp. 4639–4642, doi:10.1002/anie.200806203, PMID 19455533
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Jinzhu Chen, Kegui Li, Limin Chen, Ruliang Liu, Xing Huang (2014), "Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal–organic frameworks", Green Chem. (in German), vol. 16, no. 5, pp. 2490–2499, doi:10.1039/C3GC42414F, ISSN 1463-9262
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Christophe Volkringer, Seth M. Cohen (2010-06-21), "Generating Reactive MILs: Isocyanate- and Isothiocyanate-Bearing MILs through Postsynthetic Modification", Angewandte Chemie International Edition (in German), vol. 49, no. 27, pp. 4644–4648, doi:10.1002/anie.201001527, PMID 20480478
- ^ a b c d e Andrea Centrone, Takuya Harada, Scott Speakman, T. Alan Hatton (2010-07-07), "Facile Synthesis of Vanadium Metal-Organic Frameworks and their Magnetic Properties", Small (in German), vol. 6, no. 15, pp. 1598–1602, doi:10.1002/smll.201000773, PMID 20623532
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Alexis S. Munn, Franck Millange, Michel Frigoli, Nathalie Guillou, Clément Falaise (2016), "Iodine sequestration by thiol-modified MIL-53(Al)", CrystEngComm (in German), vol. 18, no. 41, pp. 8108–8114, doi:10.1039/C6CE01842D, ISSN 1466-8033
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b Shyam Biswas, Sarah Couck, Dmytro Denysenko, Asamanjoy Bhunia, Maciej Grzywa (2013-11-15), "Sorption and breathing properties of difluorinated MIL-47 and Al-MIL-53 frameworks", Microporous and Mesoporous Materials (in German), vol. 181, pp. 175–181, doi:10.1016/j.micromeso.2013.07.030
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Claudia Zlotea, Delphine Phanon, Matjaz Mazaj, Daniela Heurtaux, Vincent Guillerm (2011), "Effect of NH2 and CF3 functionalization on the hydrogen sorption properties of MOFs", Dalton Transactions (in German), vol. 40, no. 18, p. 4879, doi:10.1039/c1dt10115c, ISSN 1477-9226, PMID 21431158
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ a b c Karen Markey, Martin Krüger, Tomasz Seidler, Helge Reinsch, Thierry Verbiest (2017-11-16), "Emergence of Nonlinear Optical Activity by Incorporation of a Linker Carrying the p -Nitroaniline Motif in MIL-53 Frameworks", The Journal of Physical Chemistry C (in German), vol. 121, no. 45, pp. 25509–25519, doi:10.1021/acs.jpcc.7b09190, ISSN 1932-7447, PMC 5694968, PMID 29170688
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Christophe Volkringer, Thierry Loiseau, Nathalie Guillou, Gérard Férey, Mohamed Haouas (2010-10-05), "High-Throughput Aided Synthesis of the Porous Metal−Organic Framework-Type Aluminum Pyromellitate, MIL-121, with Extra Carboxylic Acid Functionalization", Inorganic Chemistry (in German), vol. 49, no. 21, pp. 9852–9862, doi:10.1021/ic101128w, ISSN 0020-1669, PMID 20923169
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Morgane Sanselme, Jean-Marc Grenèche, Myriam Riou-Cavellec, Gérard Férey (August 2004), "The first ferric carboxylate with a three-dimensional hydrid open-framework (MIL-82): its synthesis, structure, magnetic behavior and study of its dehydration by Mössbauer spectroscopy", Solid State Sciences (in German), vol. 6, no. 8, pp. 853–858, Bibcode:2004SSSci...6..853S, doi:10.1016/j.solidstatesciences.2004.04.001
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Christian Serre, Franck Millange, Thomas Devic, Nathalie Audebrand, Wouter Van Beek (2006-08-10), "Synthesis and structure determination of new open-framework chromium carboxylate MIL-105 or CrIII(OH)·{O2C–C6(CH3)4–CO2}·nH2O", Materials Research Bulletin (in German), vol. 41, no. 8, pp. 1550–1557, doi:10.1016/j.materresbull.2006.01.013
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Angiolina Comotti, Silvia Bracco, Piero Sozzani, Satoshi Horike, Ryotaro Matsuda (2008-10-15), "Nanochannels of Two Distinct Cross-Sections in a Porous Al-Based Coordination Polymer", Journal of the American Chemical Society (in German), vol. 130, no. 41, pp. 13664–13672, doi:10.1021/ja802589u, ISSN 0002-7863, PMID 18798624
{{citation}}: CS1 maint: multiple names: authors list (link) - ^ Mendt, Matthias; Jee, Bettina; Himsl, Dieter; Moschkowitz, Lutz; Ahnfeldt, Tim; Stock, Norbert; Hartmann, Martin; Pöppl, Andreas (March 2014). "A Continuous-Wave Electron Paramagnetic Resonance Study of Carbon Dioxide Adsorption on the Metal–Organic Frame-Work MIL-53". Applied Magnetic Resonance. 45 (3): 269–285. doi:10.1007/s00723-014-0518-6. ISSN 0937-9347. S2CID 94965421.
- ^ Mendt, Matthias; Jee, Bettina; Stock, Norbert; Ahnfeldt, Tim; Hartmann, Martin; Himsl, Dieter; Pöppl, Andreas (2010-11-18). "Structural Phase Transitions and Thermal Hysteresis in the Metal−Organic Framework Compound MIL-53 As Studied by Electron Spin Resonance Spectroscopy". The Journal of Physical Chemistry C. 114 (45): 19443–19451. doi:10.1021/jp107487g. ISSN 1932-7447.
- ^ Barth, Benjamin; Mendt, Matthias; Pöppl, Andreas; Hartmann, Martin (2015-11-01). "Adsorption of nitric oxide in metal-organic frameworks: Low temperature IR and EPR spectroscopic evaluation of the role of open metal sites". Microporous and Mesoporous Materials. Special Issue: New Generations of Porous Metal-Organic Frameworks. 216: 97–110. doi:10.1016/j.micromeso.2015.02.020. ISSN 1387-1811.
- ^ Kozachuk, Olesia; Meilikhov, Mikhail; Yusenko, Kirill; Schneemann, Andreas; Jee, Bettina; Kuttatheyil, Anusree V.; Bertmer, Marko; Sternemann, Christian; Pöppl, Andreas; Fischer, Roland A. (2013-09-03). "A Solid-Solution Approach to Mixed-Metal Metal-Organic Frameworks - Detailed Characterization of Local Structures, Defects and Breathing Behaviour of Al/V Frameworks". European Journal of Inorganic Chemistry. 2013 (26): 4546–4557. doi:10.1002/ejic.201300591.
- ^ Nevjestić, Irena; Depauw, Hannes; Gast, Peter; Tack, Pieter; Deduytsche, Davy; Leus, Karen; Van Landeghem, Melissa; Goovaerts, Etienne; Vincze, Laszlo; Detavernier, Christophe; Van Der Voort, Pascal (2017). "Sensing the framework state and guest molecules in MIL-53(Al) via the electron paramagnetic resonance spectrum of V IV dopant ions". Physical Chemistry Chemical Physics. 19 (36): 24545–24554. Bibcode:2017PCCP...1924545N. doi:10.1039/C7CP04760F. hdl:1854/LU-8534452. ISSN 1463-9076. PMID 28852751.
- ^ Osadchii, Dmitrii Y.; Olivos-Suarez, Alma I.; Szécsényi, Ágnes; Li, Guanna; Nasalevich, Maxim A.; Dugulan, Iulian A.; Crespo, Pablo Serra; Hensen, Emiel J. M.; Veber, Sergey L.; Fedin, Matvey V.; Sankar, Gopinathan (June 2018). "Isolated Fe Sites in Metal Organic Frameworks Catalyze the Direct Conversion of Methane to Methanol". ACS Catalysis. 8 (6): 5542–5548. doi:10.1021/acscatal.8b00505. hdl:10754/627902. ISSN 2155-5435. S2CID 104144811.
- ^ Bignami, Giulia P. M.; Davis, Zachary H.; Dawson, Daniel M.; Morris, Samuel A.; Russell, Samantha E.; McKay, David; Parke, Richard E.; Iuga, Dinu; Morris, Russell E.; Ashbrook, Sharon E. (2018). "Cost-effective 17 O enrichment and NMR spectroscopy of mixed-metal terephthalate metal–organic frameworks". Chemical Science. 9 (4): 850–859. doi:10.1039/C7SC04649A. ISSN 2041-6520. PMC 5873045. PMID 29629152.
- ^ Depauw, Hannes; Nevjestić, Irena; De Winne, Jonatan; Wang, Guangbo; Haustraete, Katrien; Leus, Karen; Verberckmoes, An; Detavernier, Christophe; Callens, Freddy; De Canck, Els; Vrielinck, Henk (2017). "Microwave induced "egg yolk" structure in Cr/V-MIL-53". Chemical Communications. 53 (60): 8478–8481. doi:10.1039/C7CC04651K. ISSN 1359-7345. PMID 28703241.
- ^ Nouar, Farid; Devic, Thomas; Chevreau, Hubert; Guillou, Nathalie; Gibson, Emma; Clet, Guillaume; Daturi, Marco; Vimont, Alexandre; Grenèche, Jean Marc; Breeze, Matthew I.; Walton, Richard I. (2012). "Tuning the breathing behaviour of MIL-53 by cation mixing". Chemical Communications. 48 (82): 10237–10239. doi:10.1039/c2cc35348b. ISSN 1359-7345. PMID 22968060.
- ^ Breeze, Matthew I.; Clet, Guillaume; Campo, Betiana C.; Vimont, Alexandre; Daturi, Marco; Grenèche, Jean-Marc; Dent, Andrew J.; Millange, Franck; Walton, Richard I. (2013-07-15). "Isomorphous Substitution in a Flexible Metal–Organic Framework: Mixed-Metal, Mixed-Valent MIL-53 Type Materials" (PDF). Inorganic Chemistry. 52 (14): 8171–8182. doi:10.1021/ic400923d. ISSN 0020-1669. PMID 23815225. S2CID 40656565.
- ^ Sun, Qiao; Liu, Min; Li, Keyan; Han, Yitong; Zuo, Yi; Chai, Fanfan; Song, Chunshan; Zhang, Guoliang; Guo, Xinwen (2017-01-13). "Synthesis of Fe/M (M = Mn, Co, Ni) bimetallic metal organic frameworks and their catalytic activity for phenol degradation under mild conditions". Inorganic Chemistry Frontiers. 4 (1): 144–153. doi:10.1039/C6QI00441E. ISSN 2052-1553.
- ^ Marx, Stefan; Kleist, Wolfgang; Huang, Jun; Maciejewski, Marek; Baiker, Alfons (2010). "Tuning functional sites and thermal stability of mixed-linker MOFs based on MIL-53(Al)". Dalton Transactions. 39 (16): 3795–8. doi:10.1039/c002483j. ISSN 1477-9226. PMID 20372702.
- ^ Lescouet, Tristan; Kockrick, Emanuel; Bergeret, Gérard; Pera-Titus, Marc; Aguado, Sonia; Farrusseng, David (2012). "Homogeneity of flexible metal–organic frameworks containing mixed linkers". Journal of Materials Chemistry. 22 (20): 10287. doi:10.1039/c2jm15966j. ISSN 0959-9428.
- ^ Lescouet, Tristan; Kockrick, Emanuel; Bergeret, Gerard; Pera-Titus, Marc; Farrusseng, David (2011). "Engineering MIL-53(Al) flexibility by controlling amino tags". Dalton Transactions. 40 (43): 11359–61. doi:10.1039/c1dt11700a. ISSN 1477-9226. PMID 21975376.
- ^ Pera-Titus, M.; Lescouet, T.; Aguado, S.; Farrusseng, D. (2012-05-03). "Quantitative Characterization of Breathing upon Adsorption for a Series of Amino-Functionalized MIL-53". The Journal of Physical Chemistry C. 116 (17): 9507–9516. doi:10.1021/jp2117856. ISSN 1932-7447.
- ^ Yang, Jie; Yan, Xing; Xue, Teng; Liu, Yongshen (2016). "Enhanced CO 2 adsorption on Al-MIL-53 by introducing hydroxyl groups into the framework". RSC Advances. 6 (60): 55266–55271. Bibcode:2016RSCAd...655266Y. doi:10.1039/C6RA09350G. ISSN 2046-2069.
- ^ Andonova, Stanislava; Ivanova, Elena; Yang, Jie; Hadjiivanov, Konstantin (2017-08-31). "Adsorption Forms of CO 2 on MIL-53(Al) and MIL-53(Al)–OH x As Revealed by FTIR Spectroscopy". The Journal of Physical Chemistry C. 121 (34): 18665–18673. doi:10.1021/acs.jpcc.7b05538. ISSN 1932-7447.
