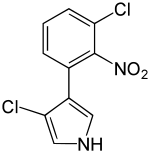
Pyrrolnitrin
Appearance
| |
| Names | |
|---|---|
| IUPAC name
3-chloro-4-(3-chloro-2-nitrophenyl)-1H-pyrrole
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.012.557 |
| EC Number |
|
| KEGG | |
| MeSH | D011764 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H6Cl2N2O2 | |
| Molar mass | 257.07284 |
| Pharmacology | |
| D01AA07 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pyrrolnitrin is an antifungal antibiotic.[1] Pseudomonas pyrrocinia and other Pseudomonas species produce pyrrolnitrin from tryptophan as secondary metabolite.[2][3] The fungicides fenpiclonil and fludioxonil are chemically related to pyrrolnitrin.[4][5]
References
- ^ Gordee, R. S.; Matthews, T. R. (1969). "Systemic antifungal activity of pyrrolnitrin". Applied Microbiology. 17 (5): 690–694. PMC 377781. PMID 5785951.
- ^ Zhu, X.; Van Pee, K. -H.; Naismith, J. H. (2010). "The Ternary Complex of PrnB (the Second Enzyme in the Pyrrolnitrin Biosynthesis Pathway), Tryptophan, and Cyanide Yields New Mechanistic Insights into the Indolamine Dioxygenase Superfamily". Journal of Biological Chemistry. 285 (27): 21126–21133. doi:10.1074/jbc.M110.120485. PMC 2898318. PMID 20421301.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Park, J. Y.; Oh, S. A.; Anderson, A. J.; Neiswender, J.; Kim, J. -C.; Kim, Y. C. (2011). "Production of the antifungal compounds phenazine and pyrrolnitrin from Pseudomonas chlororaphis O6 is differentially regulated by glucose". Letters in Applied Microbiology. 52 (5): 532–537. doi:10.1111/j.1472-765X.2011.03036.x. PMID 21362001.
- ^ Pillonel, Ch; Knauf-beiter, G.; Steinemann, A. (2003). "Encyclopedia of Agrochemicals". doi:10.1002/047126363X.agr106. ISBN 047126363X.
{{cite journal}}:|chapter=ignored (help); Cite journal requires|journal=(help) - ^ Jespers, A.B.K.; Davidse, L.C.; Dewaard, M.A. (1993). "Biochemical Effects of the Phenylpyrrole Fungicide Fenpiclonil in Fusarium sulphureum (Schlecht)". Pesticide Biochemistry and Physiology. 45 (2): 116–129. doi:10.1006/pest.1993.1014. ISSN 0048-3575.
