Peroxydisulfate

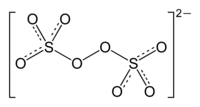
The peroxydisulfate ion, S
2O2−
8, is an oxyanion. It is commonly referred to as the persulfate ion or peroxodisulfate anions,[1] but this term also refers to the peroxomonosulfate ion, SO2−
5. Approximately 500,000 tons of salts containing this anion are produced annually. Important salts include sodium persulfate (Na2S2O8), potassium persulfate (K2S2O8), and ammonium persulfate ((NH4)2S2O8). These salts are colourless, water-soluble solids that are strong oxidants.[2]
Applications
Salts of peroxydisulfate are mainly used to initiate the polymerization of various alkenes, including styrene, acrylonitrile, and fluoroalkenes. Polymerization is initiated by the homolysis of the peroxydisulfate:
- [O3SO–OSO3]2− ⇌ 2 [SO4]•−
Moreover, sodium peroxydisulfate can be used for soil and groundwater remediation, water and wastewater treatment, and etching of copper on circuit boards.[3][1]
It has also been used to produce hair lighteners and bleaches, medical drugs, cellophane, rubber, soaps, detergents, adhesive papers, dyes for textiles, and in photography.[1]
In addition to its major commercial applications, peroxydisulfate participates in reactions of interest in the laboratory:
- Elbs persulfate oxidation
- Oxidation of Ag+ to Ag2+
References
- ^ a b c Shafiee, Saiful Arifin; Aarons, Jolyon; Hamzah, Hairul Hisham (2018). "Electroreduction of Peroxodisulfate: A Review of a Complicated Reaction". Journal of the Electrochemical Society. 165 (13). ECS: H785–H798. doi:10.1149/2.1161811jes.
- ^ Harald Jakob, Stefan Leininger, Thomas Lehmann, Sylvia Jacobi, Sven Gutewort. "Peroxo Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ^ Wacławek, S., Lutze, H. V., Grübel, K., Padil, V.V.T., Černík, M., Dionysiou, D.D. (2017) (2017). "Chemistry of persulfates in water and wastewater treatment: A review". Chemical Engineering Journal. 330: 44–62. doi:10.1016/j.cej.2017.07.132.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link)
