Dexpramipexole
| |
| Names | |
|---|---|
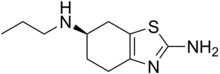
| IUPAC name
(R)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H17N3S | |
| Molar mass | 211.33 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dexpramipexole (KNS-760704) is a drug that was investigated by Knopp Biosciences and Biogen Idec for the treatment of amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease. The drug failed to show efficacy in terms of function or survival in a Phase III study of patients with ALS.[1]
More recently, dexpramipexole was shown in a Phase 2 open-label trial to reduce eosinophil counts and eliminate the need for corticosteroid treatment in a subset of patients with steroid-responsive hypereosinophilic syndrome.[2] Knopp Biosciences is advancing the drug in both HES and eosinophilic asthma.
In September 2009, dexpramipexole received Fast Track designation for ALS from the U.S. Food and Drug Administration (FDA).[3] It has received Orphan Drug designation by both the FDA and the European Medicines Agency. Dexpramipexole is a low molecular weight, water-soluble, orally bioavailable, renally excreted compound with linear pharmacokinetics, and has been shown to be well tolerated in human subject testing.[4]
KNS-760704 is the enantiomer of pramipexole, and has been shown to improve mitochondrial function and to confer significant cellular protection in neurons under stress.[5] KNS-760704 was originally identified as a candidate therapy for ALS by James Bennett, M.D., Ph.D., then of the University of Virginia.
A 2010 Phase II clinical trial involving 102 patients showed a slowing of ALS disease progression.[6]
In January 2013, Biogen Idec announced that it was discontinuing its development of dexpramipexole in ALS due to lack of efficacy in a Phase III study.[1]
See also
- Pramipexole, the racemic mixture of dexpramipexole
References
- ^ a b "Biogen Idec Reports Top-Line Results from Phase 3 Trial Investigating Dexpramipexole in People with Amyotrophic Lateral Sclerosis (ALS)". Biogen Idec. Archived from the original on 22 January 2013. Retrieved 4 January 2013.
- ^ http://www.bloodjournal.org/content/128/22/1327?sso-checked=true
- ^ FDA Fast Track KNS-760704 for ALS, September 2, 2009
- ^ Safety, Tolerability, and Pharmacokinetics of KNS-760704 (Dexpramipexole) in Healthy Adult Subjects. Michael E. Bozik, James L. Mather, William G. Kramer, Valentin K. Gribkoff and Evan W. Ingersoll. J Clin Pharmacol. Published online 19 October 2010.
- ^ KNS-760704 [(6R)-4,5,6,7-tetrahydro-N6-propyl-2, 6-benzothiazole-diamine dihydrochloride monohydrate] for the Treatment of Amyotrophic Lateral Sclerosis. Valentin K. Gribkoff and Michael E. Bozik. CNS Neuroscience & Therapeutics 14 (2008) 215–226.
- ^ Robinson, Richard (2010). "New Als Drug Shows Dose-Dependent Efficacy in Phase 2 Trial". Neurology Today. 10 (13): 1. doi:10.1097/01.NT.0000384108.10957.21.
External links
- STATEMENT ON A NONPROPRIETARY NAME ADOPTED BY THE USAN COUNCIL, contains structural formula and chemical names
