8-Oxogeranial
Appearance
This article provides insufficient context for those unfamiliar with the subject. (February 2015) |
| |
| Names | |
|---|---|
| IUPAC name
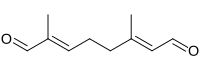
(2E,6E)-2,6-Dimethyl-2,6-octadienedial
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H14O2 | |
| Molar mass | 166.220 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
8-Oxogeranial, also incorrectly called 10-oxogeranial, is a monoterpene.[1] The terpenoid is produced by 8-hydroxygeraniol dehydrogenase which uses 8-hydroxygeraniol as its substrate. 8-Oxogeranial is itself a substrate for iridoid synthase which synthesizes cis–trans-iridodial and cis–trans-nepetalactol.[2]
References
- ^ Dewick (2009) Medicinal Natural Products: A Biosynthetic Approach.
- ^ Miettinen, Karel; Dong, Lemeng; Navrot, Nicolas; Schneider, Thomas; Burlat, Vincent; Pollier, Jacob; Woittiez, Lotte; Van Der Krol, Sander; Lugan, Raphaël; Ilc, Tina; Verpoorte, Robert; Oksman-Caldentey, Kirsi-Marja; Martinoia, Enrico; Bouwmeester, Harro; Goossens, Alain; Memelink, Johan; Werck-Reichhart, Danièle (2014). "The seco-iridoid pathway from Catharanthus roseus". Nature Communications. 5: 3606. Bibcode:2014NatCo...5.....M. doi:10.1038/ncomms4606. PMC 3992524. PMID 24710322.
