Dehydration reaction
In chemistry, a dehydration reaction is a conversion that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction. Common dehydrating agents used in organic synthesis include sulfuric acid and alumina. Often dehydration reactions are effected with heating.
Dehydration reactions
The classic example of a dehydration reaction is the Fischer esterification, which involves treating a carboxylic acid with an alcohol in the presence of a dehydrating agent:
- RCO2H + R′OH ⇌ RCO2R′ + H2O
Two monosaccharides, such as glucose and fructose, can be joined together (to form sucrose) using dehydration synthesis. The new molecule, consisting of two monosaccharides, is called a disaccharide.
The process of hydrolysis is the reverse reaction, meaning that the water is recombined with the two hydroxyl groups and the disaccharide reverts to being monosaccharides.
In the related condensation reaction water is released from two different reactants.
In organic synthesis, there are many examples of dehydration reaction, for example dehydration of alcohols or sugars.
| Reaction | General equation | Examples |
|---|---|---|
| Conversion of two alcohols to an ether (substitution) | 2 R–OH → R–O–R + H2O | |
| Conversion of an acid and an alcohol to an ester (Fischer–Speier esterification) | R−COOH + R'−OH → R−COO−R' + H2O | |
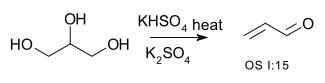
| Conversion of an alcohol to alkene (elimination) | R–CH2−CHOH–R → R–CH=CH–R + H2O | for example the conversion of glycerol to acrolein:[1]
or the dehydration of 2-methyl-1-cyclohexanol to (mainly) 1-methylcyclohexene[2], using Martin's sulfurane[3] Conversion of ethanol to ethylene[4]
|
| Conversion of two carboxylic acids an acid anhydride | 2 RCOOH → (RCO)2O + H2O | |
| Conversion of an amide to a nitrile | RCONH2 → R–CN + H2O | |
| Dienol–benzene rearrangement | 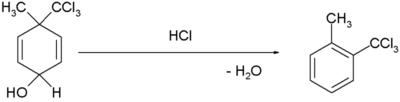 [5][6] [5][6]
|
Other examples of dehydration synthesis reactions are the formation of triglycerides from fatty acids and the formation of glycosidic bonds between carbohydrate molecules, such as the formation of maltose from two glucose molecules.
See also
References
- ^ H. Adkins; W. H. Hartung (1926). "Acrolein". Organic Syntheses. 6: 1. doi:10.15227/orgsyn.006.0001; Collected Volumes, vol. 1, p. 15.
- ^ J. Brent Friesen; Robert Schretzman (2011). "Dehydration of 2-Methyl-1-cyclohexanol: New Findings from a Popular Undergraduate Laboratory Experiment". J. Chem. Educ. 88 (8): 1141–1147. Bibcode:2011JChEd..88.1141F. doi:10.1021/ed900049b.
- ^ Roden, Brian A. (2001). "Diphenylbis(1,1,1,3,3,3-hexafluoro-2-phenyl-2-propoxy)sulfurane". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd409.
- ^ Zimmermann, Heinz; Walz, Roland (2008). "Ethylene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_045.pub3. ISBN 978-3527306732.
- ^ H. Plieninger; Gunda Keilich (1956). "Die Dienol-Benzol-Umlagerung" [The dienol-benzene rearrangement]. Angew. Chem. (in German). 68 (19): 618. doi:10.1002/ange.19560681914.
- ^ Margaret Jevnik Gentles; Jane B. Moss; Hershel L. Herzog; E. B. Hershberg (1958). "The Dienol-Benzene Rearrangement. Some Chemistry of 1,4-Androstadiene-3,17-dione". J. Am. Chem. Soc. 80 (14): 3702–3705. doi:10.1021/ja01547a058.