Leptosidin
Appearance
| |
| Names | |
|---|---|
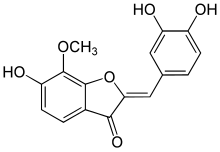
| IUPAC name
(2Z)-2-[(3,4-Dihydroxyphenyl)methylidene]-6-hydroxy-7-methoxy-1-benzofuran-3-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H12O6 | |
| Molar mass | 300.266 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Leptosidin was the first aurone to be isolated in Coreopsis grandiflora by Geissman T.A. and Heaton C.D. in 1943.[1] Leptosidin blocks the active residues of PRKACA.[2]
References
- ^ Leptosidin on metabolomics.jp
- ^ s, Sandeep; v, Priyadarshini; d, Pradhan; m, Munikumar; a, Umamaheswari (2012). "Docking and molecular dynamics simulations studies of human protein kinase catalytic subunit alpha with antagonist". Journal of Clinical and Scientific Research: 15–23. doi:10.15380/2277-5706.JCSR.12.005.
