Thianthrene

| |
| |
| Names | |
|---|---|
| IUPAC name
Thianthrene
| |
| Other names
Thianthren; 9,10-Dithiaanthracene; Di-o-phenylene disulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.998 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
| C12H8S2 | |
| Molar mass | 216.32 g·mol−1 |
| Melting point | 151 to 155 °C (304 to 311 °F; 424 to 428 K) |
| Boiling point | 364 to 366 °C (687 to 691 °F; 637 to 639 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thianthrene is a sulfur-containing heterocyclic chemical compound. It is a derivative of the parent heterocycle called dithiin. It is notable for its ease of oxidation.
Structure and synthesis
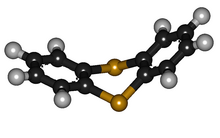
Like other 1,4-dithiins but unlike its oxygen analog dibenzodioxin, the shape of thianthrene is not planar. It is bent, with a fold angle of 128° between the two benzo groups.[2][3][4]
Thianthrene can be prepared by treating benzene with disulfur dichloride in the presence of aluminium chloride.[5]
History
Thianthrene was first synthesized by John Stenhouse by dry distillation of sodium benzenesulfonate.[6] Thianthrene is oxidized by sulfuric acid forming a red radical cation.[7] Thianthrene•+ has been characterized by Electron paramagnetic resonance. Four different publications describe the crystal structure of salts of thianthrene•+.[8]
References
- ^ Thianthrene at Sigma-Aldrich
- ^ Hosoya, S. (1963). "Molecular shapes of thianthrene and related heterocyclic compounds". Acta Crystallographica. 16 (4): 310–312. doi:10.1107/S0365110X63000797.
- ^ Gallaher, K. L.; Bauer, S. H. (1975). "Structure and inversion potential of thianthren". Journal of the Chemical Society, Faraday Transactions 2. 71: 1173–1182. doi:10.1039/F29757101173.
- ^ Aroney, M. J.; Le Fèvre, R. J. W.; Saxby, J. D. (1965). "92. Molecular polarisability. The apparent conformations of thianthren and of three of its oxides as solutes in benzene". Journal of the Chemical Society (Resumed): 571–575. doi:10.1039/JR9650000571.
- ^ US patent 3997560, "Process for the manufacture of thianthrene", issued 1976-12-14.
- ^ Stenhouse, J. (1869). "Ueber die Producte der trockenen Destillation der sulfobenzolsauren Salze" [On the Dry Distillation Products from Sulfobenzoic Acid Salts] (PDF). Annalen der Chemie und Pharmacie (in German). 149 (2): 247–255. doi:10.1002/jlac.18691490216.
- ^ W. Dilthey: Versammlungsberichte Bonner Chemische Gesellschaft, Angewandte Chemie, Volume 42, Issue 24, pp. 668–670, 15. June 1929; doi:10.1002/ange.19290422405.
- ^ Shine, Henry J. (July 1998). "EPR and the History of the Thianthrene Cation Radical". Foundations of modern EPR. ISBN 978-981-02-3295-5.
