Felinine
| |
| Names | |
|---|---|
| IUPAC name
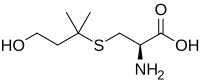
(2R)-2-Amino-3-[(3-hydroxy-1,1-dimethylpropyl)thio]propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H17NO3S | |
| Molar mass | 207.29 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Felinine, also known as (R)-2-amino-3-(4-hydroxy-2-methylbutan-2-ylthio)propanoic acid, is a chemical compound and amino acid found in cat urine and a precursor via microbial lyase of the putative cat pheromone and thiol called 3-mercapto-3-methylbutan-1-ol (MMB).[1] [2][3] Felinine is excreted by selected Felidae species including bobcats, Chinese desert cats, the kodkod, and domestic cats. Felinine synthesis occurs in the liver through a condensation reaction of glutathione and isopentenyl pyrophosphate to form 3-mercaptobutanolglutathionine (3-MBG).[4] In the kidney 3-MBG is hydrolysed and felinine partly acetylated. Cauxin assists in the hydrolysis of the dipeptide (felinylglycine) to increase the concentration of urinary felinine.[5] Urine of domestic cats may contain a series of felinine-containing compounds including free felinine, acetylfelinine, felinylglycine and 3-MBG.[6]
See also
References
- ^ Discovery of felinine: Westall, R. G. "Amino acids and other ampholytes of urine. II. Isolation of a new sulfur-containing amino acid from cat urine" Biochemical Journal (1953), 55, 244-8.
- ^ W.H. Hendriks; P.J. Moughan; M.F. Tarttelin; A.D. Woolhouse (1995). "Felinine: a urinary amino acid of Felidae". Comp. Biochem. Physiol. 112B (4): 581–588. doi:10.1016/0305-0491(95)00130-1.
- ^ P. David Josephy (28 January 2006). Molecular Toxicology. Oxford University. p. 376. ISBN 978-0-19-977145-5. Retrieved 29 July 2013.
- ^ K.J. Rutherfurd; S.M. Rutherfurd; P.J. Moughan; W.H. Hendriks (January 2002). "Isolation and Characterization of a Felinine-containing Peptide from the Blood of the Domestic Cat (Felis catus)". J. Biol. Chem. 277 (1): 114–119. doi:10.1074/jbc.M107728200. PMID 11698402.
- ^ M. Miyazaki; T. Yamashita; Y. Suzuki; Y. Saito; S. Soeta; H. Taira; A. Suzuki (October 2006). "A major urinary protein of the domestic cat regulates the production of felinine, a putative pheromone precursor". Chem. Biol. 13 (10): 1071–1079. doi:10.1016/j.chembiol.2006.08.013. PMID 17052611.
- ^ W.H. Hendriks; D.R.K. Harding; K.J. Rutherfurd-Markwick (2004). "Isolation and characterisation of renal metabolites of g-glutamylfelinylglycine in the urine of the domestic cat (Felis catus)". Comp. Biochem. Phys. 139 (2): 245–251. doi:10.1016/j.cbpc.2004.07.007. PMID 15465671.
