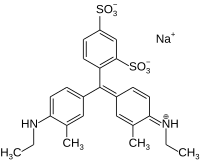
Xylene cyanol
Appearance
| |
| Names | |
|---|---|
| Other names
Acid Blue 147
xylene cyanole xylene cyanol FF xylene cyanole FF C.I. 42135 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.018.334 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C25H27N2NaO6S2 | |
| Molar mass | 538.61 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Xylene cyanol can be used as an electrophoretic color marker, or tracking dye, to monitor the process of agarose gel electrophoresis and polyacrylamide gel electrophoresis. Bromophenol blue and orange G can also be used for this purpose.
Once mixed with the sample, the concentration of xylene cyanol is typically about 0.005% to 0.03%.
Migration speed
In 1% agarose gels, xylene cyanol migrates at about the same rate as a 4 to 5 kilobase pair DNA fragment,[1] although this depends on the buffer used. Xylene cyanol on a 6% polyacrylamide gel migrates at the speed of a 140 base pair DNA fragment. On 20% denaturating (7 M urea) polyacrylamide gel electrophoresis (PAGE), xylene cyanol migrates at about the rate of 25 bases oligonucleotide.
References
- ^ Lela Buckingham and Maribeth L. Flaws (2007). Molecular Diagnostics: Fundamentals, Methods, & Clinical Applications. F.A. Davis Company. p. 91.
