Dithiolane
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dithiolane
| |||
| Other names
1,2-Dithiolane, 1,3-dithiolane
| |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C3H6S2 | |||
| Molar mass | 106.20 g·mol−1 | ||
| Related compounds | |||
Related compounds
|
1,2-Ethanedithiol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
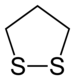
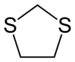
A dithiolane is a sulfur heterocycle derived from cyclopentane by replacing two methylene bridges (-CH
2- units) with thioether groups. The parent compounds are 1,2-dithiolane and 1,3-dithiolane.
1,2-Dithiolanes, such as lipoic acid or asparagusic acid, are cyclic disulfides. Some dithiolanes are natural and can be found in foods, such as asparagusic acid in asparagus. Lipoic acid has strong affinity with some metals such as gold, molybdenum, and tungsten. Other 1,2-dithiolanes have relevance in nanomaterials such as gold nanoparticles or TMDs (MoS2 and WS2).[1][2][3]
1,3-Dithiolanes are important as protecting groups for carbonyl compounds, since they are inert to a wide range of conditions. Reacting a carbonyl group with 1,2-ethanedithiol converts it to a 1,3-dithiolane, as detailed below.

References
- ^ Bilewicz, Renata; Więckowska, Agnieszka; Kruszewski, Marcin; Stępkowski, Tomasz; Męczynska-Wielgosz, Sylwia; Cichowicz, Grzegorz; Piątek, Piotr; Załubiniak, Dominika; Dzwonek, Maciej (2018-04-18). "Towards potent but less toxic nanopharmaceuticals – lipoic acid bioconjugates of ultrasmall gold nanoparticles with an anticancer drug and addressing unit". RSC Advances. 8 (27): 14947–14957. doi:10.1039/C8RA01107A. ISSN 2046-2069.
- ^ Vallan, Lorenzo; Canton-Vitoria, Ruben; Gobeze, Habtom B.; Jang, Youngwoo; Arenal, Raul; Benito, Ana M.; Maser, Wolfgang K.; D’Souza, Francis; Tagmatarchis, Nikos (2018-10-17). "Interfacing Transition Metal Dichalcogenides with Carbon Nanodots for Managing Photoinduced Energy and Charge-Transfer Processes". Journal of the American Chemical Society. 140 (41): 13488–13496. doi:10.1021/jacs.8b09204. hdl:10442/16257. ISSN 0002-7863. PMID 30222336.
- ^ Tagmatarchis, Nikos; Ewels, Christopher P.; Bittencourt, Carla; Arenal, Raul; Pelaez-Fernandez, Mario; Sayed-Ahmad-Baraza, Yuman; Canton-Vitoria, Ruben (2017-06-05). "Functionalization of MoS 2 with 1,2-dithiolanes: toward donor-acceptor nanohybrids for energy conversion". NPJ 2D Materials and Applications. 1 (1): 13. doi:10.1038/s41699-017-0012-8. ISSN 2397-7132.