Soai reaction
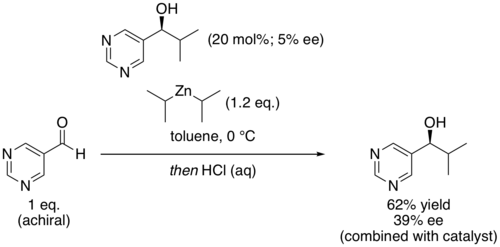
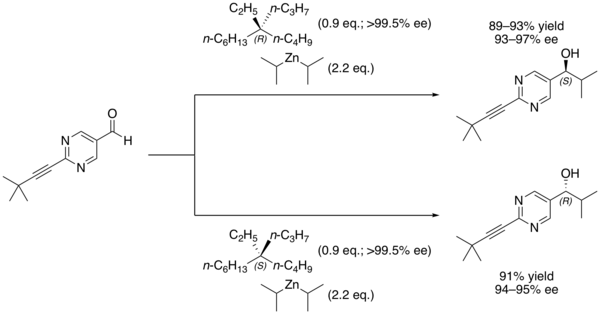
In organic chemistry, the Soai reaction is the alkylation of pyrimidine-5-carbaldehyde with diisopropylzinc. The reaction is autocatalytic and leads to rapidly increasing amounts of the same enantiomer of the product. The product pyrimidyl alcohol is chiral and induces that same chirality in further catalytic cycles. Starting with a low enantiomeric excess produces a product with very high enantiomeric excess.[1] The reaction has been studied for clues about the origin of homochirality among certain classes of biomolecules.[2]
The Japanese chemist Kenso Soai (1950–) discovered the reaction in 1995.[3][4] For his work in "elucidating the origins of chirality and homochirality", Soai received the Chemical Society of Japan award in 2010.[5]
Other chiral additives can be used as the initial source of asymmetric induction, with the major product of that first reaction being rapidly amplified. For example, Soai's group has demonstrated that even chiral quaternary hydrocarbons, which have no clear Lewis basic site for binding the nucleophile, are nonetheless capable of inducing asymmetric catalysis in the reaction.[6]
The chiral induction is believed to occur as a result of interactions between the C–H bonds of the alkane and the pi electrons of the aldehyde.[6]
References
- ^ Flügel, Rolf M. (January 11, 2011). Chirality and Life: A Short Introduction to the Early Phases of Chemical Evolution. Springer. pp. 16–17. ISBN 9783642169779.
- ^ Blackmond, D. G. (2004). "Asymmetric Catalysis Special Feature Part II: Asymmetric autocatalysis and its implications for the origin of homochirality". Proceedings of the National Academy of Sciences. 101 (16): 5732–5736. Bibcode:2004PNAS..101.5732B. doi:10.1073/pnas.0308363101. PMC 395976. PMID 15067112.
- ^ Soai, Kenso; Shibata, Takanori; Morioka, Hiroshi; Choji, Kaori (1995). "Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule". Nature. 378 (6559): 767–768. Bibcode:1995Natur.378..767S. doi:10.1038/378767a0. S2CID 4258847.
- ^ Shibata, Takanori; Morioka, Hiroshi; Hayase, Tadakatsu; Choji, Kaori; Soai, Kenso (1996). "Highly Enantioselective Catalytic Asymmetric Automultiplication of Chiral Pyrimidyl Alcohol". Journal of the American Chemical Society. 118 (2): 471–472. doi:10.1021/ja953066g.
- ^ "CSJ Award 2010: Prof. Kenso Soai". Retrieved 2015-04-14.
- ^ a b Kawasaki, T.; Tanaka, H.; Tsutsumi, T.; Kasahara, T.; Sato, I.; Soai, K. (2006). "Chiral Discrimination of Cryptochiral Saturated Quaternary and Tertiary Hydrocarbons by Asymmetric Autocatalysis". J. Am. Chem. Soc. 128 (18): 6032–6033. doi:10.1021/ja061429e. PMID 16669661.
External links
- Islas, J. R. (2005). "Mirror-symmetry breaking in the Soai reaction: A kinetic understanding". Proceedings of the National Academy of Sciences. 102 (39): 13743–13748. Bibcode:2005PNAS..10213743I. doi:10.1073/pnas.0503171102. PMC 1236534. PMID 16174731.
- Micskei, KáRoly; Rábai, Gyula; Gál, Emese; Caglioti, Luciano; Pályi, Gyula (2008). "Oscillatory Symmetry Breaking in the Soai Reaction". The Journal of Physical Chemistry B. 112 (30): 9196–200. doi:10.1021/jp803334b. PMID 18593153.
- "A Consequence of the Soai-Reaction: Re-evaluation of Racemates" (PDF).
- Podlech, Joachim; Gehring, Timo (2005). "New Aspects of Soai's Asymmetric Autocatalysis". Angewandte Chemie International Edition. 44 (36): 5776–5777. doi:10.1002/anie.200501742. PMID 16078286.