From Wikipedia, the free encyclopedia
Viniferal
Names
IUPAC name
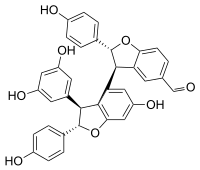
(2R ,2′S ,3R ,3′S )-3′-(3,5-Dihydroxyphenyl)-6′-hydroxy-2,2′-bis(4-hydroxyphenyl)-2,2′,3,3′-tetrahydro-[3,4′-bibenzofuran]-5-carbaldehyde
Other names
(-)-Viniferal
Identifiers
ChemSpider
InChI=1S/C35H26O8/c36-17-18-1-10-29-27(11-18)32(35(42-29)20-4-8-23(38)9-5-20)28-15-26(41)16-30-33(28)31(21-12-24(39)14-25(40)13-21)34(43-30)19-2-6-22(37)7-3-19/h1-17,31-32,34-35,37-41H/t31-,32-,34+,35-/m0/s1
Y Key: DHTHKPNODOWMKF-VPIGGYNKSA-N
Y InChI=1/C35H26O8/c36-17-18-1-10-29-27(11-18)32(35(42-29)20-4-8-23(38)9-5-20)28-15-26(41)16-30-33(28)31(21-12-24(39)14-25(40)13-21)34(43-30)19-2-6-22(37)7-3-19/h1-17,31-32,34-35,37-41H/t31-,32-,34+,35-/m0/s1
Key: DHTHKPNODOWMKF-VPIGGYNKBX
O=CC1=CC=C(O[C@@H](C2=CC=C(O)C=C2)[C@@H]3C4=CC(O)=CC5=C4[C@H](C6=CC(O)=CC(O)=C6)[C@@H](C7=CC=C(O)C=C7)O5)C3=C1
Properties
C 35 H 26 O 8
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Viniferal is a hydroxystilbenoid with an aldehyde group found in Vitis vinifera [ 1]
References
^ Ito, J (1996). "Absolute structures of new hydroxystilbenoids, vitisin C and viniferal, from Vitis vinifera 'Kyohou'Tetrahedron . 52 (30): 9991– 9998. doi :10.1016/0040-4020(96)00543-1 .
External links
Dimers Trimers Tetramers: Higher polymers Oligomeric forms
Dimers Trimers Tetramers Pentamers Hexamers Higher polymers
Glycosides or conjugates