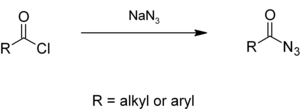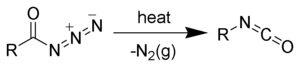Acyl azide
Appearance

Acyl azides are carboxylic acid derivatives with the general formula RCON3.
Preparation
Alkyl or aryl acyl chlorides react with sodium azide in aqueous solution to give acyl azides.[1][2]
They can also be synthesized from various carboxylic acids and sodium azide in presence of triphenylphosphine and trichloroacetonitrile catalysts in excellent yields at mild conditions.[3] Another route starts with aliphatic and aromatic aldehydes reacting with iodine azide which is formed from sodium azide and iodine monochloride in acetonitrile.[4]
Uses
Acyl azides are used as chemical reagents. On Curtius rearrangement acyl azides yield isocyanates.[5][6][7][8]
Acyl azides are also formed in Darapsky degradation,[9][10][11][12][13]
References
- ^ C. F. H. Allen and Alan Bell. "Undecyl isocyanate". Organic Syntheses; Collected Volumes, vol. 3, p. 846.
- ^ Jon Munch-Petersen (1963). "m-Nitrobenzazide". Organic Syntheses; Collected Volumes, vol. 4, p. 715.
- ^ Jang, Doo; Kim, Joong-Gon (2008). "Direct Synthesis of Acyl Azides from Carboxylic Acids by the Combination of Trichloroacetonitrile, Triphenylphosphine and Sodium Azide". Synlett. doi:10.1055/s-2008-1077979.
- ^ Marinescu, Lavinia; Thinggaard, Jacob; Thomsen, Ib B.; Bols, Mikael (2003). "Radical Azidonation of Aldehydes". The Journal of Organic Chemistry. 68 (24): 9453–5. doi:10.1021/jo035163v. PMID 14629171.
- ^ Curtius, T. (1890). Ber. 23: 3023.
{{cite journal}}: Missing or empty|title=(help) - ^ Curtius, Th. (1894). "20. Hydrazide und Azide organischer Säuren I. Abhandlung". Journal für Praktische Chemie. 50: 275. doi:10.1002/prac.18940500125.
- ^ Smith, P. A. S. (1946). Org. React. 3: 337–449.
{{cite journal}}: Missing or empty|title=(help) - ^ Scriven, Eric F. V.; Turnbull, Kenneth (1988). "Azides: Their preparation and synthetic uses". Chemical Reviews. 88 (2): 297. doi:10.1021/cr00084a001.
- ^ A. Darapsky (1936). "Darstellung von ?-Aminosäuren aus Alkyl-cyanessigsäuren". J. Prakt. Chem. 146 (8–12): 250. doi:10.1002/prac.19361460806.
- ^ A. Darapsky; D. Hillers (1915). "Über das Hydrazid der Cyanessigsäure, Isonitrosocyanessigsäure und Nitrocyanessigsäure". J. Prakt. Chem. 92: 297. doi:10.1002/prac.19150920117.
- ^ P. E. Gagnon; P. A. Boivin; H. M. Craig (1951). "Synthesis of Amino Acids from Substituted Cyanoacetic Esters". Can. J. Chem. 29: 70. doi:10.1139/v51-009.
- ^ E. H. Rodd (1965). "Chemistry of Carbon Compounds" (2nd ed.). New York: 1157.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Gagnon, Paul E.; Nadeau, Guy; Côté, Raymond (1952). "Synthesis of α-Amino Acids from Ethyl Cyanoacetate". Can. J. Chem. 30 (8): 592. doi:10.1139/v52-071.