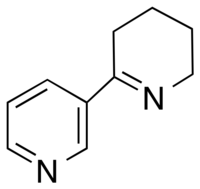
Anabaseine
| |
| Names | |
|---|---|
| Other names
3,4,5,6-Tetrahydro-2,3'-bipyridin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H12N2 | |
| Molar mass | 160.220 g·mol−1 |
| Appearance | Oil |
| Odor | Odorless |
| Boiling point | 110-120℃ |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Anabaseine (3,4,5,6-Tetrahydro-2,3’-bipyridine) is an alkaloid toxin produced by Nemertines and Aphaenogaster ants.[1] It is structurally similar to nicotine and anabasine.[2] Similarly, it has been shown to act as an agonist on most nicotinic acetylcholine receptors in the central nervous system and peripheral nervous system.[2]
Mechanism of action
The iminium form of anabaseine binds to most nicotinic acetylcholine receptors in both the peripheral nervous system and central nervous system. But, there is a higher binding affinity for receptors in the brain with a α7 subunit, as well as skeletal muscle receptors.[3] Binding causes the depolarization of neurons, and induces the release of release of both dopamine and norepinephrine.[2]
Biological effects
Anabaseine causes paralysis in crustaceans and insects, but not in vertebrates, presumably by acting as an agonist on peripheral neuromuscular nicotinic acetylcholine receptors.[2]
Structure
The anabaseine molecule consists of a non-aromatic tetrahydropyridine ring connected to the 3rd carbon of a 3-pyridyl ring. It can exist in three forms at physiological pH: a ketone, imine, or iminium structure.[2] Due to conjugation between the imine and 3-pyridyl ring, anabaseine exists as a nearly coplanar molecule.

Synthesis
Spath and Mamoli first synthesized anabaseine in 1936.[4] The researchers reacted benzoic anhydride with δ-valerolactam to yield N-benzoylpiperidone. Then, N-benzoylpiperidone is reacted with nicotinic acid ethyl ester to produce α-nicotinoyl-N-benzoyl-2-piperidone. This product then is decarboxylated, undergoes a ring closure, and amide hydrolysis to form anabaseine.

Additional synthetic strategies have since been developed by Bloom,[5] Zoltewicz,[6] Smith,[7] and Villemin.[8]
Derivatives
Due to anabaseine’s fairly non-specific binding to nicotinic acetylcholine receptors, the molecule was largely discarded as a useful tool in research or medicine. However, anabaseine derivatives have been identified with a more selective α7 binding profile. One such derivative (GTS-21, 3-(2,4-dimethoxybenzylidene)-anabaseine) has been studied as a drug candidate for cognitive and memory deficits, particularly associated with schizophrenia; it has been studied in phase II clinical trials without progression to phase III.[9]
References
- ^ Wheeler, JW; Olubajo, O; Storm, CB; Duffield, RM (6 March 1981). "Anabaseine: venom alkaloid of aphaenogaster ants". Science. 211 (4486): 1051–2. doi:10.1126/science.211.4486.1051. PMID 17744933.
- ^ a b c d e Kem, William; Soti, Ferenc; Wildeboer, Kristin; LeFrancois, Susan; MacDougall, Kelly; Wei, Dong-Qing; Chou, Kuo-Chen; Arias, Hugo R. (2006-04-06). "The Nemertine Toxin Anabaseine and Its Derivative DMXBA (GTS-21): Chemical and Pharmacological Properties". Marine Drugs. 4 (3): 255–273. doi:10.3390/md403255. Retrieved 2015-04-20.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kem, WR; Mahnir, VM; Papke, RL; Lingle, CJ (December 1997). "Anabaseine is a potent agonist on muscle and neuronal alpha-bungarotoxin-sensitive nicotinic receptors". The Journal of Pharmacology and Experimental Therapeutics. 283 (3): 979–92. PMID 9399967.
- ^ Padilla, Edited by Dean F. Martin [and] George M. (1973). Marine pharmacognosy; action of marine biotoxins at the cellular level (First ed.). New York: Academic Press. pp. 54–55. ISBN 978-0124745506.
{{cite book}}:|first1=has generic name (help) - ^ Bloom, Linda. "Influence of solvent on the ring-chain hydrolysis equilibrium of anabaseine and synthesis of anabaseine and nicotine analogues". University of Florida. Retrieved 5 May 2015.
- ^ Zoltewicz, John A.; Cruskie, Michael P. (August 1995). "A Superior Synthesis of Cholinergic Anabaseine". Organic Preparations and Procedures International. 27 (4): 510–513. doi:10.1080/00304949509458490.
- ^ Smith, Aaron. "Synthesis and Radiolabeling of Potassium Trifluoroborate Benzilidene Anabaseine Derivatives". University of Tennessee - Knoxville.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Villemin, Didier; Hachemi, Messaoud (2001). "Cesium Fluoride on Calcium Oxide as a Strongly Basic Catalyst. Synthesis of Flavones and Tobacco Alkaloids". Reaction Kinetics and Catalysis Letters. 72 (1): 3–10. doi:10.1023/A:1010597826749.
- ^ Celanire, Sylvain; Poli, Sonia (2014-10-13). Small Molecule Therapeutics for Schizophrenia. Springer. p. 248. ISBN 9783319115023. Retrieved 2015-04-20.
