Arrhenius plot
An Arrhenius plot displays the logarithm of kinetic constants (, ordinate axis) plotted against inverse temperature (, abscissa). Arrhenius plots are often used to analyze the effect of temperature on the rates of chemical reactions. For a single rate-limited thermally activated process, an Arrhenius plot gives a straight line, from which the activation energy and the pre-exponential factor can both be determined.
| Example: Nitrogen dioxide decay |
|---|
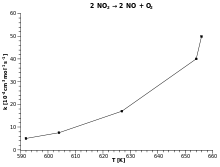 k against T |
 ln(k) against 1/T |
The Arrhenius equation can be given in the form:
or alternatively
The only difference is the energy units: the former form uses energy/mole, which is common in chemistry, while the latter form uses energy directly, which is common in physics. The different units are accounted for in using either = Gas constant or Boltzmanns constant .
The former form can be written equivalently as:
-
- Where:
When plotted in the manner described above, the value of the "y-intercept" will correspond to , and the gradient of the line will be equal to .
The pre-exponential factor, A, is a constant of proportionality that takes into account a number of factors such as the frequency of collision between and the orientation of the reacting particles.
The expression represents the fraction of the molecules present in a gas which have energies equal to or in excess of activation energy at a particular temperature.














