Arsonic acid
Appearance
| Names | |
|---|---|
| IUPAC names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AsH3O3 | |
| Molar mass | 125.943 g·mol−1 |
| Conjugate base | Hydrogen arsonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
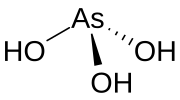

Arsonic acid is the simplest of the arsonic acids. It is a hypothetical compound, although the tautomeric arsenious acid (As(OH)3) is well established. In contrast to the instability of HAsO(OH)2, the phosphorus compound with analogous stoichiometry exists as the tetrahedral tautomer. Similarly, organic derivatives such as phenylarsonic acid are tetrahedral with pentavalent central atom.[4]
There are similar acids that are the same except for having different pnictogens. The phosphorus equivalent is phosphonic acid.
References
- ^ "Arsonic acid | H3AsO3 | ChemSpider". www.chemspider.com. Retrieved 9 October 2018.
Arsonic acid [ACD/Index Name] [ACD/IUPAC Name]
- ^ "Arsonic acid". pubchem.ncbi.nlm.nih.gov. Retrieved 9 October 2018.
IUPAC Name dihydroxy(oxo)arsenic
- ^ Munoz-Hernandez, M.-A. (1994). "Arsenic: Inorganic Chemistry". In King, R. B. (ed.). Encyclopedia of Inorganic Chemistry. Chichester: John Wiley & Sons.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
