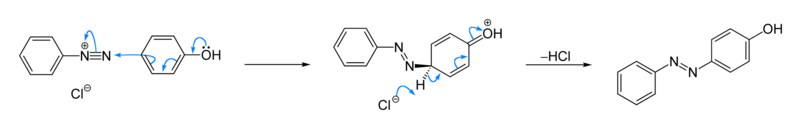
Azo coupling
An azo coupling is an organic reaction between a diazonium compound and a dialkylaniline (C6H5NR2), phenol or other aromatic compound which produces an azo compound [1] [2] . In this reaction the diazonium salt is an electrophile and the activated arene is a nucleophile in an electrophilic aromatic substitution. In most cases, including the example below, the diazonium compound is also aromatic. The product will absorb longer wavelengths of light than the reactants because of increased conjugation. Aromatic azo compounds tend to be brightly colored due to the extended conjugated systems; many are used as dyes.
Azo couplings are important in the production of dyes and pH indicators such as methyl red and pigment red 170. Prontosil and other sulfa drugs are also produced using this reaction.
An example is the synthesis of the dye organol brown from aniline and 1-naphthol:
References
- ^ J. L. Hartwell and Louis F. Fieser. "Coupling of o-tolidine and Chicago acid". Organic Syntheses; Collected Volumes, vol. 2, p. 145.
- ^ H. T. Clarke and W. R. Kirner. "Methyl red". Organic Syntheses; Collected Volumes, vol. 1, p. 374.