BQ-123
Appearance
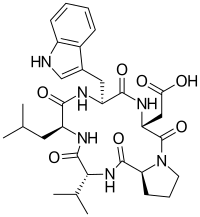
| |
| Names | |
|---|---|
| IUPAC name
2-[(3R,6R,9S,12R,15S)-6-(1H-indol-3-ylmethyl)-9-(2-methylpropyl)-2,5,8,11,14-pentaoxo-12-propan-2-yl-1,4,7,10,13-pentazabicyclo[13.3.0]octadecan-3-yl]acetic acid
| |
| Other names
Cyclo(D-trp-D-asp-L-pro-D-val-L-leu)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C31H42N6O7 | |
| Molar mass | 610.712 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
BQ-123 is a cyclic peptide consisting of five amino acids. The amino acid sequence is D-tryptamine-D-aspartic acid-L-proline-D-valine-L-leucine.
BQ-123 is a selective ETA endothelin receptor antagonist.[1][2] As such, it is used as a biochemical tool in the study of endothelin receptor function.
References
- ^ a b BQ-123 at Sigma-Aldrich
- ^ Ishikawa, Kiyofumi; Fukami, Takehiro; Nagase, Toshio; Fujita, Kagari; Hayama, Takashi; Niiyama, Kenji; Mase, Toshiaki; Ihara, Masaki; Yano, Mitsuo (1992). "Cyclic pentapeptide endothelin antagonists with high ETA selectivity. Potency- and solubility-enhancing modifications". Journal of Medicinal Chemistry. 35 (11): 1239–42.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
