Baeyer's reagent
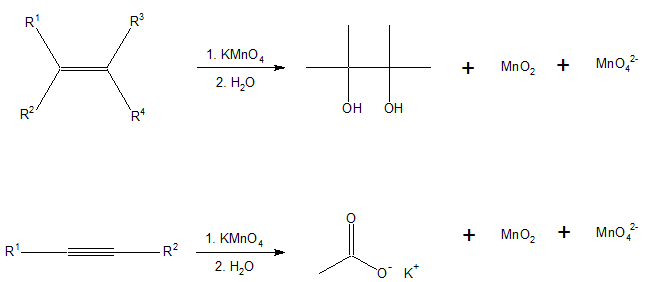
Baeyer's reagent, named after the German organic chemist Adolf von Baeyer, is used in organic chemistry as a qualitative test for the presence of unsaturation, such as double or triple bonds.

Baeyer's reagent is an alkaline solution of cold potassium permanganate, which is a powerful oxidant making this a redox reaction. Reaction with double or triple bonds (-C=C- or -C≡C-) in an organic material causes the color to fade from purplish-pink to brown. It is a syn addition reaction. Aldehydes and formic acid (and formic acid esters) also give a positive test.[1]
See also
- Bromine test, another test for the presence of unsaturation
- Iodine test, another test for presence of saturation
