Barbituric acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
pyrimidine-2,4,6(1H,3H,5H)-trione
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.598 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H4N2O3 | |||
| Molar mass | 128.087 g·mol−1 | ||
| Appearance | White crystals | ||
| Melting point | 245 °C (473 °F; 518 K) | ||
| Boiling point | 260 °C (500 °F; 533 K) | ||
| 142 g/l (20 °C) | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active.
Synthesis
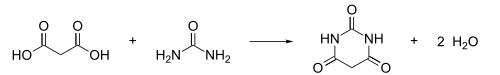
The compound was discovered by the German chemist Adolf von Baeyer on December 4, 1864, the feast of Saint Barbara (who gave the compound its namesake), by combining urea and malonic acid in a condensation reaction.[1] Malonic acid has since been replaced by diethyl malonate,[2] as using the ester avoids the problem of having to deal with the acidity of the carboxylic acid and its unreactive carboxylate.
Properties
The α-carbon has a reactive hydrogen atom and is quite acidic (pKa = 4.01) even for a diketone species (cf. dimedone with pKa 5.23 and acetylacetone with pKa 8.95) because of the additional aromatic stabilization of the carbanion.
Uses
Using the Knoevenagel condensation reaction, barbituric acid can form a large variety of barbiturate drugs that behave as central nervous system depressants. As of 2007, more than 2550 barbiturates and related compounds have been synthesised, with 50 to 55 in clinical use around the world at present. The first to be used in medicine was barbital (Veronal) starting in 1903, and the second, phenobarbital was first marketed in 1912.
Barbituric acid is one of four ingredients used to make riboflavin (vitamin B2).
Health and safety
Overdose of barbituric acid can cause respiratory problems and death.[citation needed]
See also
References
- ^ Baeyer, Adolf (1864). "Untersuchungen über die Harnsäuregruppe". Annalen der Chemie und Pharmacie. 131 (3): 291. doi:10.1002/jlac.18641310306.
- ^ J. B. Dickey & A. R. Gray (1943). "Barbituric acid". Organic Syntheses; Collected Volumes, vol. 2, p. 60.
Mahmudov K.T., Kopylovich M.N., Maharramov A.M., Kurbanova M.M., Gurbanov A.V., Pombeiro A.J.L. Barbituric acids as a useful tool for the construction of coordination and supramolecular compounds, Coordination Chemistry Reviews, 2014, 265, 1-37. DOI: 10.1016/j.ccr.2014.01.002 http://www.sciencedirect.com/science/article/pii/S0010854514000046
Mahmudov K.T., Kopylovich M.N., Maharramov A.M., Kurbanova M.M., Gurbanov A.V., Pombeiro A.J.L. Barbituric acids as a useful tool for the construction of coordination and supramolecular compounds, Coordination Chemistry Reviews, 2014, 265, 1-37. DOI: 10.1016/j.ccr.2014.01.002 http://www.sciencedirect.com/science/article/pii/S0010854514000046