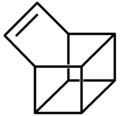
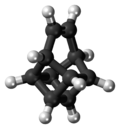
Basketene
Appearance
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Pentacyclo[4.4.0.02,5.03,8.04,7]dec-9-ene
| |||
| Other names
Bishomocubene
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H10 | |||
| Molar mass | 130.186 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Basketene (IUPAC name: pentacyclo[4.4.0.02,5.03,8.04,7]dec-9-ene[1]) is an organic compound with the formula C10H10. It is a polycyclic alkene and the dehydrogenated version of basketane, which was named for its structural similarity to a basket.
Synthesis
Basketene has been synthesized by the isomerization of cyclooctatetraene followed by a Diels–Alder reaction with maleic anhydride. [2 + 2] cycloaddition closes the cage structure, which is converted to basketene by saponification and decarboxylation.[2]
Reactions
Photolysis of basketene produces cyclooctatetraene, Nenitzescu's hydrocarbon and so on.[3]
References
- ^ Verevkin, Sergei P.; Martin Kümmerlin; Ernst Hickl; Hans-Dieter Beckhaus; Christoph Rüchardt; Sergei I. Kozhushkov; Rainer Haag; Roland Boese; Jordi Benet-Bucholz; Karsten Nordhoff; Armin de Meijere (2002). "Thermochemical and X-ray Crystallographic Investigations of Some (CH)10 Hydrocarbons: Basketene, Nenitzescu's Hydrocarbon, and Snoutene". European Journal of Organic Chemistry. 2002 (14). Wiley VCH: 2280–7. doi:10.1002/1099-0690(200207)2002:14<2280::AID-EJOC2280>3.0.CO;2-R. Archived from the original on 2013-01-05. Retrieved 2009-11-23.
- ^ Smit, William A.; Bochkov, Alekseĭ Feodosʹevich; Caple, Ron (1998). Organic synthesis: the science behind the art. Royal Society of Chemistry. p. 184. ISBN 978-0-85404-544-0. Retrieved 2008-12-10.
- ^ Allred, Evan L.; Beck, Boyd R. (1973). "Concurrent thermolysis and photolysis of basketene. Formation and the interrelation of some new (CH)10 isomers". Journal of the American Chemical Society. 95 (7): 2393. doi:10.1021/ja00788a065.