Cell cortex
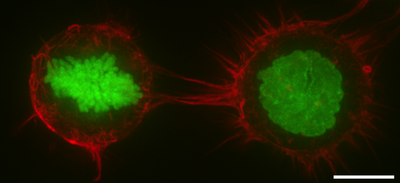
The cell cortex, also known as the actin cortex or actomyosin cortex, is a specialized layer of cytoplasmic protein on the inner face of the plasma membrane of the cell periphery. It functions as a modulator of plasma membrane behavior and cell surface properties.[1][2][3] In most eukaryotic cells lacking a cell wall, the cortex is an actin-rich network consisting of F-actin filaments, myosin motors, and actin-binding proteins.[4][5] The actomyosin cortex is attached to the cell membrane via membrane-anchoring proteins called ERM proteins and it plays a central role in cell shape control.[1][6] The protein constituents of the cortex undergo rapid turnover, making the cortex both mechanically rigid and highly plastic, two properties essential to its function. In most cases, the cortex is in the range of 100 to 1000 nanometers thick.
In some animal cells, the protein spectrin may be present in the cortex. Spectrin helps to create a network by cross-linked actin filaments.[3] The proportions of spectrin and actin vary with cell type.[7] Spectrin proteins and actin microfilaments are attached to transmembrane proteins by attachment proteins between them and the transmembrane proteins. The cell cortex is attached to the inner (cytosolic) face of the plasma membrane in cells where the spectrin proteins and actin microfilaments form a mesh-like structure much like a fishnet except that it can be broken and reformed.
In plant cells, the cell cortex is reinforced by cortical microtubules underlying the plasma membrane. The direction of these cortical microtubules determines which way the cell elongates when it grows.
Functions
- In mitosis, F-actin and myosin II form a highly contractile and uniform cortex to drive mitotic cell rounding. The surface tension produced by the actomyosin cortex activity generates intracellular hydrostatic pressure capable of displacing surrounding objects to facilitate rounding.[8][9]
- In cytokinesis the cell cortex plays a central role by producing a myosin-rich contractile ring to constrict the dividing cell into two daughter cells.[10]
- Cell cortex contractility is key for amoeboidal type cell migration characteristic of many cancer cell metastasis events.[1][11]
References
- ^ a b c Salbreux, Guillaume; Charras, Guillaume; Paluch, Ewa (October 2012). "Actin cortex mechanics and cellular morphogenesis". Trends in Cell Biology. 22 (10): 536–545. doi:10.1016/j.tcb.2012.07.001.
- ^ Pesen D, Hoh JH (January 2005). "Micromechanical architecture of the endothelial cell cortex". Biophys. J. 88 (1): 670–9. doi:10.1529/biophysj.104.049965. PMC 1305044. PMID 15489304.
- ^ a b Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter (2002). "Cross-linking Proteins with Distinct Properties Organize Different Assemblies of Actin Filaments". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 0-8153-3218-1.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ "The evolution of compositionally and functionally distinct actin filaments". Journal of Cell Science. 128 (11): 2009–19. 2015. doi:10.1242/jcs.165563. PMID 25788699.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Clark, Andrew G; Wartlick, Ortrud; Salbreux, Guillaume; Paluch, Ewa K (19 May 2014). "Stresses at the Cell Surface during Animal Cell Morphogenesis". Current Biology. 24 (10): R484–R494. doi:10.1016/j.cub.2014.03.059.
- ^ Fehon, Richard G.; McClatchey, Andrea I.; Bretscher, Anthony (2010-04-01). "Organizing the cell cortex: the role of ERM proteins". Nature Reviews Molecular Cell Biology. 11 (4): 276–287. doi:10.1038/nrm2866. PMC 2871950. PMID 20308985.
- ^ Machnicka B, Grochowalska R, Bogusławska DM, Sikorski AF, Lecomte MC (January 2012). "Spectrin-based skeleton as an actor in cell signaling". Cell. Mol. Life Sci. 69 (2): 191–201. doi:10.1007/s00018-011-0804-5. PMC 3249148. PMID 21877118.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stewart, Martin P; Helenius, Jonne; Toyoda, Yusuke; Ramanathan, Subramanian; Muller, Daniel J; Hyman, Anthony A (2 January 2011). "Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding". Nature. 469: 226–230. doi:10.1038/nature09642. PMID 21196934.
- ^ Ramanathan, Subramanian; Helenius, Jonne; Stewart, Martin P; Cattin, Cedric; Hyman, Anthony A; Muller, Daniel J (26 January 2015). "Cdk1-dependent mitotic enrichment of cortical myosin II promotes cell rounding against confinement". Nature Cell Biology. 17: 148–159. doi:10.1038/ncb3098. PMID 25621953.
- ^ Green, Rebecca A; Paluch, Ewa K; Oegema, Karen (November 2012). "Cytokinesis in animal cells". Annual Review of Cell and Developmental Biology. 28: 29–58. doi:10.1146/annurev-cellbio-101011-155718.
- ^ Olson, M.F.; Sahai, Eric (April 2009). "The actin cytoskeleton in cancer cell motility". Clinical and Experimental Metastasis. 29 (4): 273–287. doi:10.1007/s10585-008-9174-2.
Further reading
