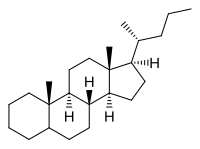
Cholane
Appearance
| |
| Names | |
|---|---|
| IUPAC name
(5S,8R,9S,10S,13R,14S,17R)-10,13-Dimethyl-17-[(1R)-1-methylbutyl]-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H42 | |
| Molar mass | 330.59 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
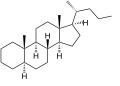
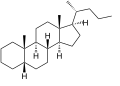
Cholane is a steroid with a molecular weight of 330.59. It can exist as either of two stereoisomers, 5α-cholane and 5β-cholane.
-
5α-Cholane
-
5β-Cholane
See also
External links
- Cholanes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Bellini A, Mencini E, Quaglio M, Guarneri M, Fini A (1991). "Antimicrobial activity of basic cholane derivatives. X. Synthesis of 3 alpha- and 3 beta-amino-5 beta-cholan-24-oic acids". Steroids. 56 (7): 395–398. doi:10.1016/0039-128X(91)90073-5. PMID 1780957.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Fini A, Fazio G, Roda A, Bellini A, Mencini E, Guarneri M (1992). "Basic cholane derivatives. XI: Comparison between acid and basic derivatives". J Pharm Sci. 81 (7): 726–730. doi:10.1002/jps.2600810728. PMID 1403713.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Roda A, Bellini A, Mencini E, Minutello A, Fini A, Guarneri M (1992). "Effect of basic cholane derivatives on intestinal cholic acid metabolism: in vitro and in vivo activity". J Pharm Sci. 81 (3): 237–240. doi:10.1002/jps.2600810310. PMID 1640360.
{{cite journal}}: CS1 maint: multiple names: authors list (link)