Crown ether

Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer (n = 4), the pentamer (n = 5), and the hexamer (n = 6). The term "crown" refers to the resemblance between the structure of a crown ether bound to a cation, and a crown sitting on a head. The first number in a crown ether's name refers to the number of atoms in the cycle, and the second number refers to the number of those atoms that are oxygen. Crown ethers are much broader than the oligomers of ethylene oxide; an important group are derived from catechol.
Crown ethers strongly bind certain cations, forming complexes. The oxygen atoms are well situated to coordinate with a cation located at the interior of the ring, whereas the exterior of the ring is hydrophobic. The resulting cations often form salts that are soluble in nonpolar solvents, and for this reason crown ethers are useful in phase transfer catalysis. The denticity of the polyether influences the affinity of the crown ether for various cations. For example, 18-crown-6 has high affinity for potassium cation, 15-crown-5 for sodium cation, and 12-crown-4 for lithium cation. The high affinity of 18-crown-6 for potassium ions contributes towards its toxicity.
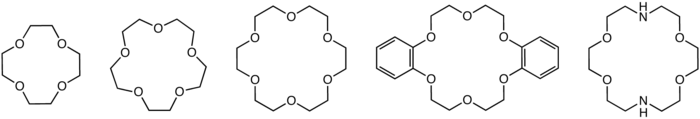
structures of common crown ethers: 12-crown-4, 15-crown-5, 18-crown-6, dibenzo-18-crown-6, and diaza-18-crown-6
Crown ethers in nature
Crown ethers are not the only macrocyclic ligands that have affinity for the potassium cation. Ionophores such as valinomycin also display a marked preference for the potassium cation over other cations.
History of synthetic crown ethers
In 1967, Charles Pedersen, who was a chemist working at DuPont, discovered a simple method of synthesizing a crown ether when he was trying to prepare a complexing agent for divalent cations.[1][2] His strategy entailed linking two catecholate groups through one hydroxyl on each molecule. This linking defines a polydentate ligand that could partially envelop the cation and, by ionization of the phenolic hydroxyls, neutralize the bound dication. He was surprised to isolate a by-product that strongly complexed potassium cation. Citing earlier work on the dissolution of potassium in 16-crown-4,[3][4] he realized that the cyclic polyethers represented a new class of complexing agents that were capable of binding alkali metal cations. He proceeded to report systematic studies of the synthesis and binding properties of crown ethers in a seminal series of papers. The fields of organic synthesis, phase transfer catalysts, and other emerging disciplines benefited from the discovery of crown ethers. Pedersen particularly popularized the dibenzo crown ethers.[5]
Pedersen shared the 1987 Nobel Prize in Chemistry for the discovery of the synthetic routes to, and binding properties of, crown ethers.
Affinity for cations
Apart from its high affinity for potassium cations, 18-crown-6 can also bind to protonated amines and form very stable complexes in both solution and the gas phase. Some amino acids, such as lysine, contain a primary amine on their side chains. Those protonated amino groups can bind to the cavity of 18-crown-6 and form stable complexes in the gas phase. Hydrogen-bonds are formed between the three hydrogen atoms of protonated amines and three oxygen atoms of 18-crown-6. These hydrogen-bonds make the complex a stable adduct.
Aza-crowns
Some or all of the oxygen atoms in crown ethers can be replaced by nitrogens to form cryptands. A well-known tetrazacrown is cyclen in which there are no oxygens. [6]
See also
References
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ja01002a035, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ja01002a035instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ja00986a052, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ja00986a052instead. - ^ D. G. Stewart. D. Y. Waddan and E. T. Borrows, GB 785229 Oct. 23, 1957.
- ^ J. L. Down, J. Lewis, B. Moore and G. W. Wilkinson, Proc. Chem. Soc., 1959, 209; J. Chem. Soc., 1959, 3767.
- ^ Charles J. Pedersen (1988). "Macrocyclic Polyethers: Dibenzo-18-Crown-6 Polyether and Dicyclohexyl-18-Crown-6 Polyether". Organic Syntheses; Collected Volumes, vol. 6, p. 395.
- ^ Vincent J. Gatto, Steven R. Miller, and George W. Gokel (1988). "4,13-Diaza-18-Crown-6". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 8, p. 152.

