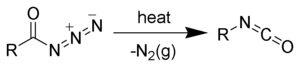Curtius rearrangement
The Curtius rearrangement (or Curtius reaction or Curtius degradation), as first defined by Theodor Curtius, is a chemical reaction that involves the rearrangement of an acyl azide to an isocyanate.[1][2] Several reviews have been published.[3][4]
The isocyanate can be trapped by a variety of nucleophiles. Water is often added in order to hydrolyze the isocyanate to an amine.[5] When done in the presence of tert-butanol, the reaction generates Boc-protected amines, useful intermediates in organic synthesis.[6][7]
Carboxylic acids 1 can be easily converted to acyl azides 3 using diphenylphosphoryl azide 2.[8][9][10]
Likewise, when the Curtius reaction is performed in the presence of benzyl alcohol, Cbz-protected amines are formed.[11]
Reaction mechanism
The Curtius rearrangement may be thought of as a two-step process, the first step being the loss of nitrogen gas, forming an acyl nitrene (2), and the second step being the rearrangement of acyl nitrenes by migration of R-group to form the desired isocyanate (3). However, current evidence indicates that these two steps are likely concerted (i.e., they occur at the same time), and no free nitrene intermediate is formed.[12]
Scope
In one variation called the Darapsky degradation (A. Darapsky, 1936), a Curtius rearrangement takes place as one of the steps from an α-cyanoester to an amino acid.[13]
See also
- Beckmann rearrangement
- Hofmann rearrangement
- Lossen rearrangement
- Schmidt reaction
- Tiemann rearrangement
- Wolff rearrangement
References
- ^ Curtius, T. (1890). Ber. 23: 3023.
{{cite journal}}: Missing or empty|title=(help) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1002/prac.18940500125, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1002/prac.18940500125instead. - ^ Smith, P. A. S. (1946). Org. React. 3: 337–449.
{{cite journal}}: Missing or empty|title=(help) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/cr00084a001, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/cr00084a001instead. - ^ Kaiser, C.; Weinstock, J. (1988). "Amines from mixed carboxylic-carbonic anhydrides: 1-phenylcyclopentylamine". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 6, p. 910. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/op970115w, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/op970115winstead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ol051428b, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ol051428binstead. - ^ Shioiri, T.; Yamada, S. (1990). "Diphenyl phosphorazidate". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 7, p. 206. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ja00772a052, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ja00772a052instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S0040-4020(01)97352-1, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S0040-4020(01)97352-1instead. - ^ Jessup, P. J.; Petty, C. B.; Roos, J.; Overman, L. E. (1988). "1-N-Acylamino-1,3-dienes from 2,4-pentadienoic acids by the Curtius rearrangement: benzyl trans-1,3-butadiene-1-carbamate". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 6, p. 95. - ^ Smith, Michael B.; March, Jerry (2007). March's Advanced Organic Chemistry (6th ed.). Hoboken, New Jersey: Wiley. p. 1609. ISBN 978-0-471-72091-1.
- ^ http://www.chempensoftware.com/reactions/RXN051.htm