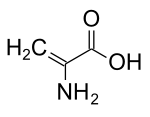
Dehydroalanine
| |
| |
| Names | |
|---|---|
| IUPAC name
2-Aminoprop-2-enoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H5NO2 | |
| Molar mass | 87.08 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dehydroalanine (Cα,β-didehydroalanine, (alpha)-(beta)-di-dehydroalanine, or 2,3-didehydroalanine) is a dehydroamino acid. It is an α-amino acid found as a residue in peptides of microbial origin. It is unusual in having an unsaturated backbone.[1]
Structure and reactivity
As a primary enamine, dehydroalanine is unstable with respect to tautomerization. It therefore can only exist as a residue in a peptide.
Because of its unsaturated side chain, dehydroalanine only exists as a single isomer. Despite its name, it is not derived from alanine. Instead it arises via a post transcriptional modification of serine and cysteine. These amino acids, as residues in peptides and proteins, undergo enzymatical loss of water and hydrogen sulfide, respectively.
Generally amino acid residues are unreactive toward nucleophiles, but those containing dehydroalanine (like some other dehydroamino acids) are exceptions.[1]
Occurrence
Dehydroalanine was first detected in the cyclic antimicrobial peptide nisin.[1]
Dehydroalanine (DHA) is also found in food proteins, including casein, that have been heated in the presence of a base such as sodium hydroxide. Bases can dehydrate serine to form DHA.[citation needed]
In food, DHA frequently alkylates lysine to yield the reactive lysinoalanine or N6-(DL-2-amino-2-carboxyethyl)-L-lysine, an unusual amino acid thought to cause kidney failure in rats, has been found in proteins of home-cooked and commercial foods and ingredients. Although it had been reported to occur in both edible and non-food proteins only after base treatment (such as occurs in soybean processing), it has also been identified in food proteins that had not been subjected to treatment. Lysinoalanine can also be generated in a variety of proteins when heated under non-alkaline conditions.
Many dehydroalanine-containing peptides are toxic or antibiotic. DHA is a component of lantibiotics and microcystins.
See also
References
- ^ a b c Dawid Siodłak "α,β-Dehydroamino Acids in Naturally Occurring Peptides" Amino Acids 2015, vol. 47, pp. 1–17. doi:10.1007/s00726-014-1846-4.
CID 123991 from PubChem
