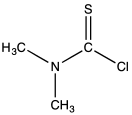
Dimethylthiocarbamoyl chloride
Appearance
| |
| Identifiers | |
|---|---|
PubChem CID
|
|
| Properties | |
| C3H6ClNS | |
| Molar mass | 123.60 g·mol−1 |
| Appearance | yellow solid |
| Melting point | 39–43 °C (102–109 °F; 312–316 K) |
| Boiling point | 90–95 °C (194–203 °F; 363–368 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dimethylthiocarbamoyl chloride is an organosulfur compound with the formula (CH3)2NC(S)Cl. A yellow solid, it is often encountered as a yellow syrup. It is a key reagent in the synthesis of arylthiols via the Newman-Kwart rearrangement.[1]
Synthesis and reactions
Representative of other thiocarbamoyl chlorides, dimethylthiocarbamoyl chloride is electrophilic, serving as a source of R2NC(S)+.[2] It is analogous to dimethylcarbamoyl chloride (R2NC(O)Cl).
Dimethylthiocarbamoyl chloride is prepared by chlorination of the related tetramethylthiuram disulfide:
- [Me2NC(S)]2S2 + 3 Cl2 → 2 Me2NC(S)Cl + 2 SCl2
Dimethylthiocarbamoyl chloride reacts with dithiocarbamates (R2NCS−
2) to give thiuram sulfides [R2NC(S)]2S. With methanethiolate, it gives methyl dimethyldithiocarbamate (Me2NC(S)SMe).
References
- ^ "Thiophenols from Phenols: 2-Naphthalenethiol". Org. Synth. 51: 139. 1971. doi:10.15227/orgsyn.051.0139.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ R. J. Cremlyn “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). ISBN 0 471 95512 4
